COLUMN: SCIENCE FOR FORMULATORS
Soap and Syndet
Abstract
Recently, consumer’s interest in natural, and sustainable cosmetics have turned their attention to soap technology. The evaluation in both new and old technology has opened up an interest in both soap and syndet bars. In the famous song “All That Jazz, Peter Allen reminds us that “Everything old Is New Again” (1) . He admonishes: Don't throw the past away, you might need it some rainy day. That rainy day is on the horizon.
This article will explain the difference between soap and syndet. There is a growing interest and natural soap and hybrid natural soap due to growing as consumers demand. Whilst there are still some draw backs to “simple natural soap”, many formulators are investigating new hybrid natural soap formulations that overcome some challenges.
Luis Spitz, the “Godfather of soap” states: Since its appearance in history, soap has helped safeguard two of our greatest treasures: our health and our children. Health is directly related to cleanliness. Data proves that the higher the soap consumption in a country, the lower the infant mortality rate will be. In industrialized countries, soap is the most taken for granted and readily available personal care product used on the body daily. Soap is also the most inexpensive product we use in relation to its use cost. In many developing countries, both laundry and toilet soaps remain scarce, expensive essentials (2).
It is believed that the Phoenicians were the first to develop soap making into an art. in ~1000 CE; from there, its use and manufacture spread throughout Europe.
In the 9th century CE, Marseilles, France was already famous for soap making.

Marseille Soap
In southeastern France, Provence is a region of the Camargue in which olive oil, salt,
and soda ash were readily available for soap making. In the 16th century, Marseille
became the first official soap producing region in France. The soap had to contain 72% vegetable oils. Only pure olive oil was allowed. The gentle handling of clothes and the hands made them so popular that by the 1880s, there were close to 100 Marseille soap producers in France.
Synthetic detergents took over the market and the Marseille soap for laundering
practically disappeared. During the last decade Marseille soaps, a name remembered and associated with quality soaps of the past, were rediscovered due to the rise in interest in vegetable-based, natural products.
Soap making was one of the first trades in early America. The chore of soap making
fell to women. Surplus animal fats and oils were saved to make soap 2–3 times
a year. The fats and oils were melted in a large iron kettle, which hung on a rod
over a fire; lye was mixed in using a long-handled iron spoon (3).
Soap Benefits
Soap is a surfactant. It has an oil soluble alkyl chain and a carboxyl group in the same molecule. This type of molecule (soap) when present at low concentration in water, has the property of adsorbing onto the surfaces or interfaces of the system altering surface or interfacial free energies of those surfaces (or interfaces) (3). When oil is added to the water / surfactant/ soap combination, cleansing properties result. A self-assembling (micelluar) translucent product is achieved. The resulting complex when properly chosen, can be washed away. Figure 1 (4)containsa cartoon representation of a micelle.
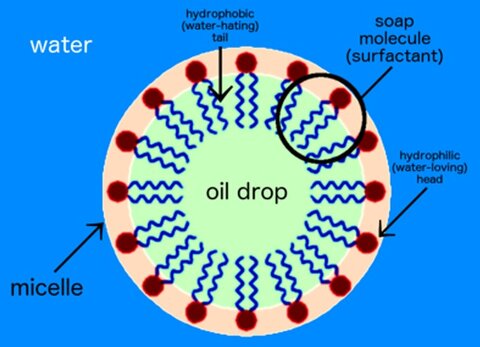
Figure 1. Soap, oil and water micelle (5).
Natural soap is derived from organic, sustainable, natural ingredients, most importantly triglycerides (for example olive oil) , and base (for example KOH or NaOH). Figure 2 shows that soap added to water will form self-assembling units. As the micelles grow in size they become larger and reflect more laser light.
Natural Soap Chemistry
(a) Soap Raw Material - Oils, Fats, Waxes, and Butters
The terms oils, fats, butters and waxes have been misused over the years. Butters, oils and fats are all triglycerides. Fats have a titer point of over 40.5 ° C, oils have a titer point of below 40.5 ° C. Butters have a titer below 40.5 ° C but above 20 ° C (6).
(b) Saponification of Triglycerides
The major process by which soap is prepared is called saponification. In this process fats, oils and triglycerides are reacted with base to produce soap, glycerin. Figure 3 shows the reaction.

Figure 2. Tips for Formulators.
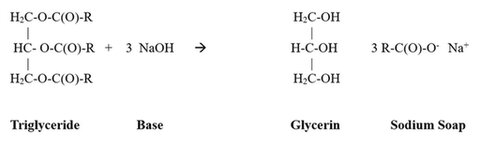
Figure 3. Saponification of Triglycerides Reaction Scheme.
When a triglyceride is saponified (reacted with a base) to make a soap, glycerin is liberated. Saponification is a general term to define the chemical reaction that breaks the ester linkage. It is possible to produce different kinds of soap products by adding glycerin and other natural ingredients. Glycerin, produced as a by-product of saponification is water soluble and fatty insoluble.
The selection of the proper raw materials to make soap is a very important step and determines the functionality, which in turn effects consumer performance.
Triglyceride
The triglyceride of interest for personal care is plant derived, rather than animal and natural rather than synthetic. The carbon distribution (shown in the “R” group in figure 3) will be a profound effect on the soap. Additionally the amount of unsaturation ( -CH=CH-) in the triglyceride determined by iodine value (a measure of unsaturation needs to be considered.
The INCI name and melting point of the triglycerides used in the soap making process are also important. The commonly used triglycerides are olive oil, coconut oiland palm oil. Table 1 provides a more detailed list of triglycerides. The triglyceride combinations can be mixed, providing a wider range of aesthetics.
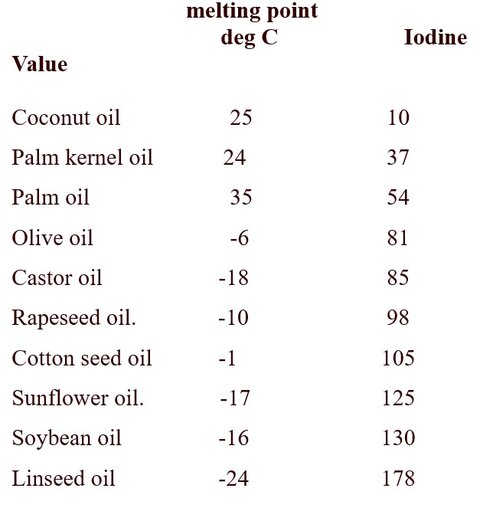
Table 1. Triglyceride Raw Materials.
Base
The choice of bases used in the saponification is also important. When sodium hydroxide (NaOH) is used in bar soap it is referred to as hard soap. When Potassium Hydroxide (KOH) is used in the saponification step the resulting soap is referred to as liquid soap. Referred to as liquid soap.

The modification of existing formulations with polymers and surfactants can result in some unexpected changes in product performance. Everything old can in fact be modified to be new again.
There are several myths that exist about properly chosen soap bars (8).
- Although some traditional soaps can be drying due to their alkaline pH and strong cleansing properties, not all regular soaps are the same. Some soaps include moisturizing ingredients such as glycerin or natural oils to help keep the skin hydrated.
- While traditional soaps can remove natural oils from the skin, this does not guarantee that they will cause dryness or irritation in everyone. For people with oily skin, removing excess oils can be beneficial. However, those with dry or sensitive skin may prefer gentler cleansing methods to avoid removing the skin's natural moisture barrier.
- While some regular soaps may contain sulphates or fragrances that can irritate sensitive skin, not all soaps are made with harsh chemicals. Many soap manufacturers provide milder options containing natural ingredients or gentle surfactants to accommodate different skin types and preferences.
- By dissolving dirt, oil, and bacteria and then washing them away with water, traditional soaps are made to effectively cleanse skin. Regular soaps can offer complete cleaning and hygiene benefits when used correctly.
- While certain soap formulations may cause irritation or dryness in some people with sensitive skin, there are milder options available that are specifically designed for sensitive skin. Additionally, fragrance-free and hypoallergenic soap varieties are often recommended for individuals with sensitivities
Chemistry - Syndet Bar
A syndet bar is not a soap bar. The term “syndet” is a combination of the words “synthetic” and “detergent.” Syndet bars are formulated using synthetic detergents instead of traditional soap, which is made from saponified fats or oils.
Syndet bars, or synthetic detergent bars, are a new type of soap-free cleansing bars which are made of a combination of different surfactants with detergency properties. Apart from impurities, syndet bars are also free from fatty acid salts, and contain only synthetic surfactants. The synthetic surfactants are generally derived from mineral oils, plant oils or animal fats of natural source, hence the syndet bars produced are known to be mild, and beneficial as personal cleansing bars or dermatological bars. Additionally, syndet bars often have a neutral pH which prevents skin irritation.
Syndet Bars ingredients include:
- Sodium lauryl sulfate
- Sodium laureth sulfate
- Sodium cocoyl isethionate
- sodium lauryl sulfoacetate
- Cocamidopropyl betaine
U.S. Patent 11,932,826 states:
The syndet bars available in the industry are generally made of sodium cocoyl isethionate. Owing to the popular use of sodium cocoyl isethionate in the manufacture of syndet bars, the price of this ingredient has also gone exorbitant in the industry. There are a number of technologies existing in the art relating to syndet bar compositions made of sodium cocoyl isethionate. For example, U.S. Pat. No. 5,691,287 discloses a low irritation cleansing bar composition containing sodium cocoyl isethionate in an amount of 20% to 35% by weight of the cleansing bar. This composition also contains fatty alcohol with a relatively lower amount of fatty acid. Asides from low irritation effect, there is no disclosure in the document on the performance of the cleansing bar composition in view of its detergency or softening effect. As sodium cocoyl isethionate is used as the main ingredient, the manufacturing costs for this cleansing bar are relatively high. Besides, sodium cocoyl isethionate is also considered as a mild surfactant and may not give rise of the desired detergency (9).
CONCLUSION
One of the most important property of natural soap is the fact that it is made from natural ingredients. Natural soap uses the saponification reaction and has glycerin in the product which is a natural part of the saponification process. Natural soap is higher in pH than syndet.
Syndet bars are milder and less irritating to skin, in particular sensitive skin.
Syndet bars are formulated with a much wider selection of synthetic ingredients. Synthetic ingredients may not appeal to consumers seeking a natural product.
References and notes
- https://www.youtube.com/watch?v=Z9FfI4-oRDo
- https://cleantheworld.org/blog/honoring-the-life-of-the-godfather-of-soap-luis-spitz/
- Rosen MJ, Kunjappu JT (2012). Surfactants and Interfacial Phenomena (4th ed.). Hoboken, New Jersey: John Wiley & Sons. p. 1. ISBN978-1-118-22902-6.
- https://www.themacbath.com/blog/2016/6/27/back-to-basics-what-is-soap
- https://www.sciencedirect.com/book/9781630670658/soap-manufacturing-technology
- http://www.scientificspectator.com/documents/book%20service/Oils_of_Nature.pdf
- http://www.scientificspectator.com/documents/Olenick%20Compilation/Ch%2030%20Min%20Dis%20Tech%202.pdf
- https://www.clinikally.com/blogs/news/syndets-vs-regular-soaps-decoding-the-best-choice-for-your-skin
- U.S. Patent 11,932,826
