
Disinfection
on
Skin care
KEYWORDS
DISINFECTION,
RAPID KILL,
SODIUM BENZOATE,
3-PHENYLPROPANOL,
Multifunctionals,
Surfactants.
peer-reviewed
Common Ingredients, Impressive Synergy:
Enhancing Rapid Kill with Sodium Benzoate
LUCAS C. WEBBER1*, AMBER W. YARNELL2
*Corresponding author
1. R&D Chemist, Flavors & Fragrances Business Unit, LANXESS Corporation, Kalama, Washington, United States
2. R&D Supervisor, Flavors & Fragrances Business Unit, LANXESS Corporation, Kalama, Washington, United States
ABSTRACT: The rapid kill properties of sodium benzoate at acidic pH ranges in combination with surfactants and a multifunctional potentiator were explored. It was found that low concentrations of sodium benzoate can exhibit rapid bactericidal activity over a broader pH range than lactic and citric acids. Addition of the aromatic alcohol 3-phenylpropanol (3PP) and anionic surfactants further enhances these properties, generating remarkable synergy against a selection of bacterial species, with log reduction values and timescales that satisfy industry expectations. This study underscores the importance of pH and surfactant selection in optimizing disinfection products when using sodium benzoate or benzoic acid. These findings contribute to advancing future disinfection strategies for home and personal care (HPC), where new disinfection technologies are desired. It is vital that new disinfectant options work in harmony with modern formulation approaches, requiring ingredients that are biodegradable and identical to chemistries found in nature. This study highlights the potential of sodium benzoate to fulfil these criteria as a safe, effective, and versatile antimicrobial tool.
INTRODUCTION
Sodium benzoate (SB), a salt of benzoic acid, is a widely used preservative with a favourable environmental, health, and safety profile and a long history of safe and effective use in food and personal care. A variety of organic acids, including benzoic acid, have been previously investigated for their disinfecting properties, but their efficacy is heavily influenced by pH levels and dosage (1, 2, 3). Benzoic acid demonstrates excellent permeability across bacterial cell membranes, and can rapidly enter the cytoplasm and induce cell death in both gram-positive and gram-negative species (1, 2, 3). However, given the low water solubility of benzoic acid, sodium benzoate was chosen to be studied in acidic solutions, since benzoic acid is still the dominant species present when SB is at a pH below 4 (Figure 1) (4).
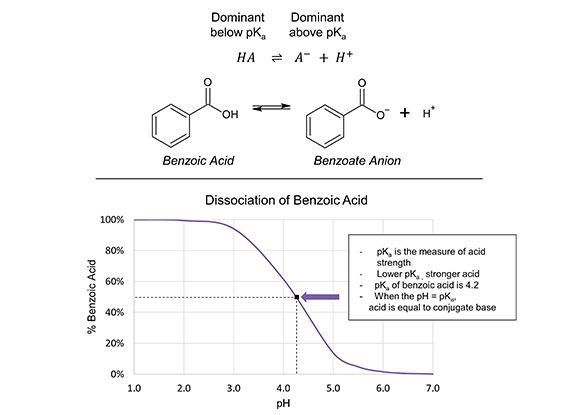
Figure 1. Top: The acid-base equilibrium of benzoic acid and the benzoate conjugate base demonstrates how, at pH ranges below the pKa of benzoic acid (4.2), the dominant species is the neutral, cell-permeable form. The acid form is largely responsible for the antibacterial properties of benzoic acid. At higher pH ranges, the deprotonated conjugate base is the dominant form, which has increased water solubility, but dramatically reduced membrane permeability. Bottom: A plot illustrating the proportion of benzoic acid as a share of total benzoate over a wide range of pH values. The distribution lies at 50:50 when the pH is equal to the pKa (4).
As microorganisms evolve, and regulatory and consumer pressure mounts against traditional disinfection chemistry, the need for alternatives that can replace or reduce conventional technologies is ever-increasing. Common chemistries in the HPC disinfection market include citric acid, lactic acid, and quaternary ammonium compounds (QACs), each with unique antimicrobial properties. Citric acid, commonly found in fruits, exhibits antimicrobial activity against a broad spectrum of microorganisms and is often used in cleaning solutions and disinfectants due to its biodegradability, low toxicity, and recognizable name (5). Lactic acid, derived from fermentation processes, also demonstrates antimicrobial properties and is frequently utilized in disinfectants for its effectiveness against bacteria, viruses, and fungi. However, both of these organic acids require very low formula pH levels and, often, high dosages (5, 6). QACs are widely used disinfectants in home care, personal care, and industrial applications, the most common being benzalkonium chloride. This class of compounds exhibit broad-spectrum antimicrobial activity, being effective against bacteria, viruses, and fungi. However, their efficacy can be affected by pH, organic matter, or other chemicals (7), highlighting the importance of understanding and optimizing disinfection formulations for maximum effectiveness.
It has also been observed that aromatic alcohols, including the fragrance multifunctional 3PP, have low minimum inhibitory concentration (MIC) values against a range of microorganisms (8) and that aromatic alcohols can boost the disinfection properties of common disinfectants (9). The authors hypothesize that these antimicrobial properties could translate into rapid kill efficacy when combined with an organic acid such as benzoic acid. This study builds upon previous research (1-3) to explore how these factors affect rapid kill efficacy, aiming to improve understanding and formulation strategies for the next generation of HPC disinfection products using established ingredients with proven value, safety, and effectiveness.
Methods and Materials
Preparation of Test Samples
All samples were prepared by dissolving each component in approximately 150 g of deionized H2O at room temperature with rapid stirring in order to achieve the desired concentration. pH adjustments were conducted by addition of 2M HCl (aq) or 10% NaOH w/w (aq) with simultaneous pH monitoring.
Rapid Kill Testing
Preparation of Inoculum: The products were tested against three microorganisms: Staphylococcus aureus (ATCC No. 6538), Salmonella enterica (ATCC No. 14028), and Escherichia coli (ATCC No. 8739). The surface of a suitable volume of solid agar medium was inoculated with a recently grown stock culture, followed by incubation at 30 - 35˚C for 24 - 48 hours, and transfer of the cultures to sterile phosphate buffered saline (PBS). The number of viable microorganisms in each milliliter of the inoculum suspensions was determined by serial dilution in PBS. The test organisms were plated at 10-6 and 10-7 dilution, and then overlayed with approximately 20 mL of 45 ˚C Tryptic Soy Agar and incubated at 30 - 35 ˚C for 24-48 hours.
Rapid Kill Evaluation: Test sample (9.9 mL) was added to a test tube, followed by aseptic addition of 0.1 mL of the test organism and thorough mixing. Each sample was allowed to sit at room temperature for the duration of each intended timepoint, at which point 1.0 mL was transferred to 9.0 mL of Dey Engley Neutralizing Broth. Serial dilutions were then performed from 10-1 to 10-5 in duplicate. A 1.0 mL aliquot of each dilution was transferred to a petri plate, which was overlayed with approximately 20 mL of 45˚C Tryptic Soy Agar before being swirled, allowed to solidify, and incubated for 48-72 hours at 30-35 ˚C. Using the calculated inoculum concentration for each test microorganism, the log reduction for each microorganism was calculated to determine kill rate, reported as log reduction (Colony-Forming Unit/mL, CFU/mL).
RESULTS
While the rapid kill properties of benzoic acid alone have been previously reported (1-3), a baseline for this work was established using samples of sodium benzoate at 0.3% w/w, acidified to pH 3.0 using HCl. When introduced to both S. aureus and S. enterica, substantial log reduction of the bacteria was observed at 5 minutes (5.81 and 3.70 log reduction, respectively) and after 10 minutes a complete kill was achieved. A control sample at low pH, containing only HCl, did not show any substantial rapid kill properties after 10 minutes (0.23 and 0.16 log CFU reduction against S. aureus and S. enterica, respectively), further suggesting that a lipophilic organic acid like benzoic acid is needed to transport protons across the cell membrane for cytoplasmic acidification and cell death to occur at short timescales.
To further examine how the structures of organic acids influence their rapid kill properties, two other common disinfectants, lactic acid and citric acid, were also studied. Illustrated below in Figure 2, it was found that when lactic acid and citric acid were tested at dosages concurrent with existing off-the-shelf products, at a pH of 3.0, these two organic acids did not demonstrate sufficient rapid kill activity when compared to benzoic acid. Surprisingly, it was found that both citric acid and lactic acid had practically no rapid kill activity at pH 3, while sodium benzoate at the same pH showed a complete kill after just 5 minutes at 15% the dosage of lactic acid against S. aureus (Figure 2). This in part could be due to the lower pKa of lactic acid and the reduced hydrophobicity (i.e. membrane permeability) of this acid compared to benzoic acid.
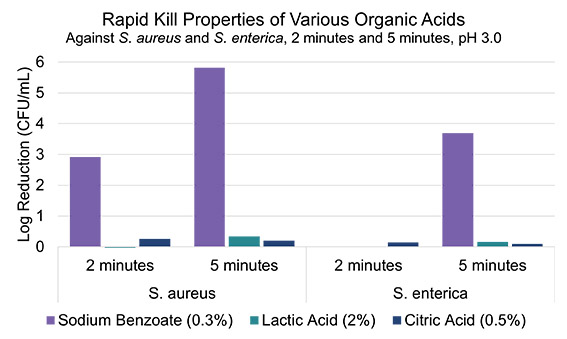
Figure 2. The rapid kill properties of sodium benzoate, lactic acid, and citric acid at pH 3.0 against two bacterial species are shown above. Only the solutions containing benzoate showed substantial efficacy against bacteria.
Next, sodium benzoate was combined with a multifunctional aromatic alcohol (3PP) that has previously demonstrated antibacterial properties and is a multifunctional that can boost preservation efficacy alongside sodium benzoate (10). At short timescales, it was found that 3PP has some activity against the gram-negative bacteria, but no activity against the gram positive alone, at pH 8. However, when combined with sodium benzoate at pH 3.0, synergy emerges against both S. aureus and S. enterica (Figure 3). It is hypothesized that 3-phenylpropanol can permeabilize bacterial membranes allowing for easier diffusion of benzoic acid into the cell, increasing the efficacy of bacterial kill (11).
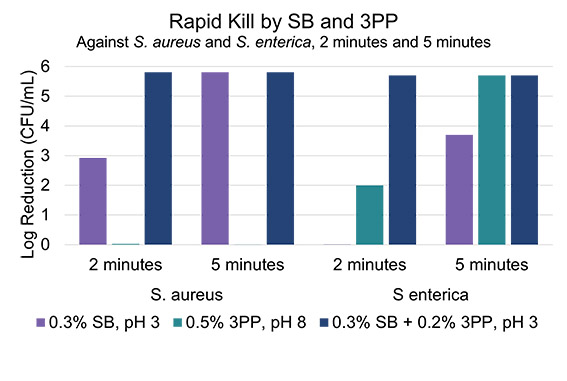
Figure 3. Log reduction of solutions containing sodium benzoate, 3-phenylpropanol, and a combination of both against S. aureus and S. enterica. The screening of performance against E. coli was not performed in this study.
To better understand what concentrations of benzoic acid are needed for rapid kill activity, a series of samples at various pH levels were tested for rapid kill activity against S. aureus and S. enterica, alongwith the addition of E. coli to further expand the scope of this study. The results are described in Figure 4. A clear dependency on pH is observed, as there a rapid drop off in efficacy above a pH of 3.5 (Figure 4).
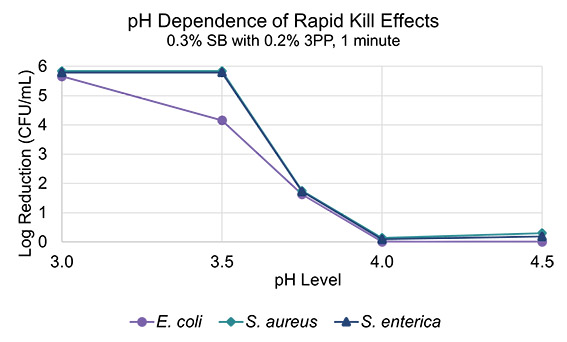
Figure 4. The pH dependence of the disinfection properties of a blend of sodium benzoate with 3-phenylpropanol.
A well-studied class of HPC ingredients that possess antimicrobial properties are surfactants. Ubiquitous options such as sodium lauryl sulphate and sodium laureth sulphate have been extensively studied, but with increasing consumer stigma against “sulphates”, alternative surfactants with greater consumer appeal and increasing rates of adoption are of interest and were investigated for rapid kill potentiation. It is known that anionic surfactants tend to have greater antimicrobial activity than amphoteric or non-ionic varieties (12), so the selection included two anionic surfactants that have widespread industry use but have not been extensively studied for disinfection properties. These surfactants included sodium lauroyl methyl isethionate (SLMI, anionic), cocoamidopropyl betaine (CAPB, amphoteric), and sodium cocoyl methyl taurate (SCMT, anionic). As such, it was found that only the anionic surfactants were able to boost the rapid kill properties of the SB/3PP blend (Figure 5), with SCMT generating the greatest amount of synergy due to its lower intrinsic disinfectant properties. Log reductions of 1% surfactant solutions in absence of SB/3PP for E. coli, S. aureus, and S. enterica are 4.60, 4.18, 1.36 for SLMI and 1.19, -0.08, and 0.27 for SMCT, respectively (Figure 5).
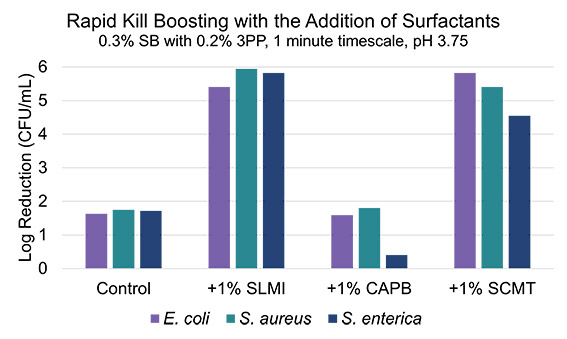
Figure 5. Demonstration of the boosting observed in rapid kill by the addition of surfactants to a solution of SB (0.3%) and 3PP (0.2%) at pH 3.75 after 1 minute. The changes in log reduction are shown, with the anionic surfactants demonstrating greater synergy than the amphoteric surfactant, CAPB.
CONCLUSION
These results contribute to the understanding of formulation strategies for developing effective disinfectant products using organic acids. It has been demonstrated that benzoic acid has disinfection properties alone at low dosages and moderately acidic pH ranges, and that it is able to outperform other common organic acids at these dosages and pH ranges. The addition of 3-phenylpropanol generates notable synergy at low levels (0.2%) when combined with benzoic acid against both gram-positive and gram-negative bacterial species. Importantly, the addition of anionic surfactants, particularly SCMT, generates remarkable disinfection synergy, achieving a greater than 4-log reduction of all three bacterial species tested at a 1-minute timescale. By optimizing pH levels and selecting appropriate surfactants, it is possible to enhance the antimicrobial properties of these compounds for various applications in the home and personal care industry. Further research is warranted to explore additional surfactant combinations and their potential synergistic effects on disinfection efficacy.
Note:
It is the responsibility of the user of this information to establish appropriate safety and health practices and determine the applicability of regulatory requirements prior to use. Antimicrobial activity as described in this article or the references therein is not a guarantee of passing antimicrobial tests required for claims substantiation. Those who wish to make such claims for products they intend to market need to consult regulatory agencies in their jurisdictions for specific requirements.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
References and notes
- Hazan R, Levine A, Abeliovich H. Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in Saccharomyces cerevisiae. Appl and Environ Microbiol. 2004;70:4449–57. Available from: https://doi.org/10.1128/AEM.70.8.4449-4457.2004.
- Chen H, Zhong Q. Antibacterial activity of acidified sodium benzoate against Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes in tryptic soy broth and on cherry tomatoes. Int J Food Microbiol. 2018;274:38–44. Available from: https://doi.org/10.1016/j.ijfoodmicro.2018.03.017.
- Ricke S. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult Sci. 2003;82:632–9. Available from: https://doi.org/10.1093/ps/82.4.632.
- Section D: General Chemical. In: Weast, RC, Lide DR, Astle MJ, Beyer WH, editors. CRC handbook of chemistry and physics. 70th ed. Boca Raton: CRC Press, Inc; p. D-150-63.
- Rosli NM, Tang JY. Effect of different conditions of citric acid and acetic acid decontamination against Esherichia coli in lettuce. J Agrobiotechnology. 2018;9(1S):54–61. Available from: https://journal.unisza.edu.my/agrobiotechnology/index.php/agrobiotechnology/article/view/166/143
- Boomsma B, Bikker E, Lansdaal E, Stuut P. L-lactic acid - A safe antimicrobial for home- and personal care formulations. SOFW. 2015;10:2–5. Available from: https://www.sofw.com/en/hikashop-menu-for-categories-listing/product/399-sofw-journal-10-2015-english-online
- Frozza R, Bado C, Schneider JE, Caselles A, Filsner PH, Brum JS. Action of benzalkonium chloride in different pH. Arquivos Do Instituto Biológico. 2021;88:1–6. Available from: https://doi.org/10.1590/1808-1657001052018.
- Mackie MA, Lyall J, McBride RJ, Murray JB, Smith G. Antimicrobial properties of some aromatic alcohols. Pharm Acta Helv. 1986;61:333–6.
- Richards RM, McBride RJ. Enhancement of Benzalkonium chloride and chlorhexidine acetate activity against Pseudomonas aeruginosa by aromatic alcohols. J Pharm Sci. 1973;62:2035–7. Available from: https://doi.org/10.1002/jps.2600621232.
- Foster S, Pippine K, Proestos Jr J, Farrell B, Vaughn-Biege J. LANXESS Corporation. Antimicrobial Compositions. United States Patent 11,917,993 B2. March 5, 2024. Available from: https://patentcenter.uspto.gov/applications/16760971.
- Yano T, Miyahara Y, Morii N, Okano T, Kubota H. Pentanol and benzyl alcohol attack bacterial surface structures differently. Appl Environ Microbiol. 2015;82:402–8. Available from: https://doi.org/10.1128/AEM.02515-15.
- Falk NA. Surfactants as antimicrobials: A brief overview of microbial interfacial chemistry and surfactant antimicrobial activity. J Surfactants Deterg. 2019;22:1119–27. Available from: https://doi.org/10.1002/jsde.12293.
