Surfactants
on
Skin care

peer-reviewed
Beyond bubbles: molecules powering modern marvels
CARRIE BROWN
Senior Manager, Regulatory Affairs, Household & Commercial Products Association
ABSTRACT: In the most empirical sense, surfactants are compounds that reduce the surface tension between two substances, enabling the molecules to disperse. Though often overlooked outside the chemical world, surfactants play a fundamental role in modern manufacturing processes and are the powerhouse behind all things soap.
The history of surfactants dates back thousands of years, with early use traced to ancient times when soaps were made from natural sources like plant ashes and animal fats. In the industrial age, scientists began synthesizing surfactants, leading to the commercial production of various types of detergents and emulsifiers. Since then, ongoing research and advancements have expanded their applications across industries, leading to the development of more effective and specialized surfactants tailored for diverse applications.
Introduction
Microplastics have received great attention in the last years all over the world. This increasing interest is mainly due to their potential harmful effects on the biota and humans through the food chain or from contact with contaminated products (2, 3). Furthermore, the negative interactions are also extended to all contaminants (chemical and biological) of which microplastics can be carriers (4, 5, 6), so microplastics could be also infections or toxic chemicals vectors. It was recently estimated that 8 M tons per year of plastics are entering the marine environment, due to accidentally or deliberately dumped litter (7). The awfulness of this situation is due to the non-biodegradability of plastics items that are accumulating in the environment as fragments of decreasing size (microplastics) (7).
Microplastics show a relevant heterogeneity, in terms of chemical classification (type of polymer), size and shape, deriving from several kind of thermal and/or mechanical degradation from macroscopic plastic items (secondary microplastics), furthermore microplastics are contained as intentionally added microparticles for specific functional purposes (primary microplastics) in several kinds of products (4). According to ECHA (2019), microplastics are defined as: "particles containing solid polymers, to which additives or other substances may have been added, with dimensions between 1 nm ≤ x ≤ 5 mm (particles) or fibres with a length between 3 nm ≤ x ≤ 15 mm, with a length/diameter ratio >3”. Biodegradable polymers, polymers with water solubility > 2g/l and natural polymers (not modified except by hydrolysis) are excluded from this definition (8). An extensive definition and description of microplastics is necessary to fully understand their distribution patterns in the environment and also to allow the development of reliable and robust analytical protocols (9), therefore an appropriate description of all the characteristics of microplastics, such as size, chemical nature, colour and shape, is very relevant and useful for understanding the origin and way of distribution in the environment of these contaminants (10).
As previously mentioned, primary microplastics are those that are intentionally added to other products with a specific functional activity, for example exfoliating particles in scrubs or in detergents, opacifiers in cosmetics in cosmetics (4, 11) or glitter in make-up and nail products. Many countries are addressing the primary microplastics problem by introducing national laws to ban or restrict the intentional addition of functional microplastics into various types of products (12), see some examples in the table below (Table 1):
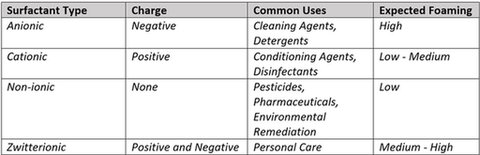
Table 1. (12) National laws to ban or restrict the intentional addition of functional microplastics.
Recently (October 2023) has been published the European Commission Regulation that insert microplastics in the XVII Allegate of REACH (Commission Regulation (EU) 2023/2055) ( ). In this context, great attention is focused on cosmetics and detergents samples, for which the ban regarding to the intentionally added microbeads is already in force and within a few years will be ban also the intentional addition of microparticles in general, firstly in rinse-off products and then also in the live-on (Commission Regulation (EU) 2023/2055)(13).
Furthermore European Commission has published a Proposal for a Regulation of the European Parliament and of the Council on preventing plastic pellet losses to reduce microplastics pollution (14).
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Earlier this year, the Utah Department of Transportation (UDOT) needed to slide a 1.1 million-pound bridge into the southbound lane of I-15(1). The crews needed to keep everything “slippery and moving” to ensure that the bridge would inch toward its final location with the proper spacing on each side. Their secret ingredient? Dish soap. In combination with the hydraulics system, crews generously applied dish soap to Teflon pads as an ingenious way to reduce friction and ensure a smooth move. This slippery function of dish soap is enabled on a molecular level by surfactants, the chemical backbone of most soaps and detergents.
Surfactants, or “surface active agents” in the most empirical sense, are compounds that reduce the surface tension between two substances, enabling the molecules to disperse(2). The history of surfactants dates back thousands of years to ancient uses where soaps were made from natural sources like plant ashes and animal fats(3). In fact, a historical Sumerian tablet from 2500 B.C. describes the oldest known procedure for man-made surfactants. This tablet could be considered the first formulation batch sheet, giving detailed instructions for quantities of oil and wood ash that needed to be mixed prior to heating. This particular soap formula was intended to wash textiles; however, Sumerians also utilized soaps for medicinal purposes. Over time, soap evolved to include a wide variety of raw materials and innovations, transitioning from craft shop production to industrial manufacture.
In the industrial age, scientists began to develop synthetic surfactants, which led to the commercial-scale production of detergents and emulsifiers. Since then, the market for surfactants has grown to a more than $40 billion industry(4), with even more potential to grow in the coming years. Ongoing research and advancement have expanded surfactant applications across various industries, leading to the development of more effective and specialized surfactants tailored for diverse uses. Modern applications of surfactants range from self-assembling nanostructures for drug delivery to key agents in the clean-up of contaminated environmental sites (2).
The phrase "like oil and water" is used to describe two entities that do not mix well or have conflicting characteristics. However, surfactants turn this phrase on its head because they possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) groups. This unique structure allows surfactants to interact at interfaces, lowering the surface tension between different phases, like oil and water, allowing them to mix.
The easiest way to describe the structure of a surfactant in an aqueous solution is by the “tadpole model.” Imagine a hydrophilic and lipophobic “head” with a hydrophobic and lipophilic “tail” in the shape of a tadpole. Variations on the size and charge of these groups can dictate the function of the surfactant, with the charge of the head group typically dictating the overall charge (2). At low concentrations, surfactants migrate to the edges of the aqueous solution but, as the concentration increases, the edges are filled and they begin to assemble into spheres called micelles. Micelles are dynamic assemblies formed by the hydrophobic surfactant tails turning inward to avoid contact with water and the hydrophilic heads facing outward to create the spherical exterior layer. Surfactant molecules organize themselves in this arrangement when they reach a specific concentration known as the critical micelle concentration (CMC)(2). Think of these micelles as tiny bubbles in the solution that range in size from 50 to 100 surfactant molecules per micelle.
Understanding micelle formation is crucial across surfactant applications and is often a key piece of formulation optimization. Micelles are not fixed in their formation and remain in a state of flux – their shape and size are determined by the way the surfactants are packed (6). If the surfactant heads are charged, they are kept apart by electrostatic repulsion, which means they will be packed more loosely. Surfactants that are not charged (or non-ionic) will pack more densely together. The addition of salt can increase the viscosity, or thickness, of a formula by reducing electrostatic repulsion between the hydrophilic heads; however, too much salt can dramatically reduce viscosity, causing the formula to fall apart. This is why many formulators create a salt curve to understand what the maximum possible viscosity is for the intended product and provide a range of salt needed to reach the desired viscosity. In addition to ionic strength and surfactant concentration, micelle stability and behavior are also determined by other factors, such as temperature, pH, and the presence of other additives.
Though often overlooked outside the chemical world, surfactants play a fundamental role in product development and modern manufacturing processes. Beyond controlling viscosity and foaming potential, surfactants are responsible for stabilizing mixtures and enhancing the efficiency of chemical reactions. Understanding these variations aids in selecting the appropriate surfactants for specific purposes, contributing significantly to the quality, consistency, and functionality of manufactured goods. Table 1 shows the different types, properties, and uses of surfactants.
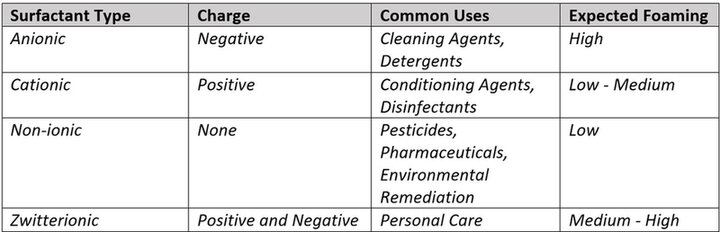
Table 1. Categorization of Surfactant (2,6).
Imagine a long list of household chores: dishwashing, floor cleaning, toilet bowl disinfecting, bathroom tile scrubbing, and loads of laundry. All these activities rely on these bubble-building powerhouses to effectively disperse oils, grime, and dirt. Their unique molecular structure enables them to surround and lift soil from surfaces or fabrics, preventing its redeposition during rinsing. Hydrophobic tails eagerly attach to grease and grime, while the hydrophilic heads cozy up to water. This partnership not only breaks down the mess but also helps lift it off surfaces, suspending it in the cleaning solution and making it easy to rinse away. Now, the real marvel? Formulations blend different types of surfactants strategically, like a recipe for the perfect potion. For example, anionic surfactants excel at cutting through grease, cationic surfactants add a soft touch, non-ionic surfactants can be used with sensitive materials, and zwitterionic surfactants bring balance, ensuring a cleaning concoction targets specific surfaces and stains.6 It's this alchemy of surfactant combinations that gives cleaning products their unique powers to handle different messes easily and effectively.
No mess is too big or too small for surfactants to tackle. In the cleanup of oil spills, surfactants are employed to disperse oil on water surfaces, breaking the oil into smaller droplets that are more readily biodegradable or can be more easily contained for removal(7). Solutions containing surfactants are then sprayed or applied to the oil slick to disperse the oil into the water column, preventing it from forming a thick surface and either aiding in its breakdown by natural processes or facilitating its recovery. This approach minimizes the environmental impact of the oil spill by preventing its spread across large surface areas and improving its biodegradation.

Stepping out of the cleaning space, surfactants play a critical role in a number of other areas. For instance, surfactants aid in metal cleaning and degreasing processes and control surface tension during soldering, which is necessary in mechanical manufacturing and electronics production(8). As previously shown in the example of salad dressing, surfactants contribute significantly to the food and beverage industries, serving as emulsifiers in dairy products, enhancing textures in baked goods, and acting as foaming agents in beer(5). They also assist in pesticide formulations for agriculture by improving the dispersion of agricultural chemicals and reducing the amount of pesticide needed. Now, how does this same substance that scrubs bathtubs and bubbles beer also save lives? Surfactants are sometimes used in pharmaceutical formulations for drug delivery, stability, and efficacy. In oral medications, surfactants like polysorbates are utilized to improve the solubility of drugs that are not necessarily water-soluble(9) by creating micellar solutions that enhance absorption in the gastrointestinal tract. Additionally, in inhalable medications, lung surfactants like dipalmitoylphosphatidylcholine (DPPC)(10) are used to improve the distribution of drugs in the lungs for effective delivery.
While the selection of surfactants is guided by considerations like stability, compatibility with food components, and compliance with rigorous regulations, it is clear that these compounds can play a number of roles across various domains.
The future of surfactants lies in innovative developments that prioritize sustainability and enhanced functionality, and immense strides have been made to develop surfactants that are made from renewable raw materials and biodegradable. Technological advancements are directing a spotlight toward optimized formulations that cater to specific needs, including tailoring surfactant structures at a molecular level to enhance their performance in various applications. Through precision engineering, scientists are creating surfactants with improved abilities to disperse oil, stabilize emulsions, and efficiently interact with different surfaces. Additionally, the design of surfactants is being optimized for specific environmental conditions, ensuring effectiveness across a range of temperatures, pH levels, and salinities.
These bubbles of ingenuity are generally responsible for keeping the world clean, tackling messes from top to bottom, but technological strides in surfactant design and application demonstrate a promising future for customized surfactants to play a pivotal role across diverse industries.
References and notes
- https://www.stgeorgeutah.com/news/archive/2022/07/18/agl-udot-moves-million-pound-bridge-in-cedar-city-with-dish-soap-and-a-bit-of-elbow-grease/
- Schramm, Laurier & Stasiuk, Elaine & Marangoni, Gerrard. (2003). Surfactants and their Applications. Annu. Rep. Prog. Chem., Sect. C: Phys. Chem. 99. 3-48. 10.1039/B208499F
- Falbe, J. (2012) Surfactants in consumer products: Theory, technology, and application. Berlin: Springer-Verlag.
- https://www.fortunebusinessinsights.com/surfactants-market-102385
- Iva Kralova & Johan Sjöblom (2009) Surfactants Used in Food Industry: A Review, Journal of Dispersion Science and Technology, 30:9, 1363-1383
- https://colonialchem.com/wp-content/uploads/2020/11/surfactants-101-4-30-20.pdf
- Kohli R. Microbial Cleaning for Removal of Surface Contamination. Developments in Surface Contamination and Cleaning. 2013:139–61.
- Zhang, L., Wang, S., Wang, T. et al. Polishing mechanisms of various surfactants in chemical mechanical polishing relevant to cobalt interconnects. Int J Adv Manuf Technol 128, 5425–5436 (2023).
- Vanti, G. Recent strategies in nanodelivery systems for natural products: a review. Environ Chem Lett 19, 4311–4326 (2021)
- Bernhard, W (November 2016). "Lung surfactant: Function and composition in the context of development and respiratory physiology". Annals of Anatomy. 208: 146–150.
