Testing
on
Skin care
peer-reviewed
Designing in vivo clinical trials for cosmetic claims
STEPHAN BIELFELDT
SGS proderm GmbH, Schenefeld, Germany
ABSTRACT: Cosmetic products are considered as fast-moving consumer goods (FMCGs), which require a fast and ever-changing adaptation of the cosmetic industry to the trends of the market. Alongside, this has an impact on how cosmetic products are presented in the market, including the product packaging, descriptions, advertising and claims. How these newly presented claims can be substantiated and what pitfalls should be considered, will be discussed in this paper.
Introduction
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Cosmetic product regulation
Cosmetic products are regulated by the CPR, the Cosmetics Products Regulation, which was implemented in its current version to simplify procedures, streamline terminology and strengthen the regulatory framework for cosmetics and to ensure a high-level of protection for human safety. The regulation additionally comprises the so-called technical document from 2017, in which it is defined how and which claims can be made. Furthermore, there are documents that discuss borderline products. These documents indicate if the product combined with the presented claim is still considered as cosmetic product, or if it might change to medical devices, medicinal products or biocides due to its presentation. Claims, in general, can be supported by different approaches: based on the ingredients used and data or references that have shown the efficacy of the respective ingredient, in vitro or ex vivo studies supporting the statements or in vivo studies on human volunteers. For the latter, there are recommendations available on how to best design an in vivo study for the claim under investigation.
With looking at all these regulations and recommendations, one might expect that it is clear to the cosmetic industry what is required and how the best approach should be. But when we look a bit closer on how to design such in vivo studies, we will quickly see the wide range of possibilities that have a clear impact on the quality of the results and, thus, the quality of the claim substantiation.
Claims and advertisement
What contributes to the claim? All that appears on the cosmetic product label, e.g. text, names, trademarks, pictures and figurative or other signs contribute to the presentation of the product and all of these have to be substantiated, unless the associated claim is a clear exaggeration or obvious for the type of the cosmetic product.
Therefore, it is not only about an advertising statement, but also about what the consumer might understand from the product presentation. Here, the cosmetic product regulation states that claims should be based on legal compliance, truthfulness, evidential support, honesty, fairness and they should allow informed decisions by the consumer. If a claim violates one of these criteria, then the claim cannot be made.
What can already be seen here is it all begins with the claim. The corresponding study, whatever type it is, can only be the most appropriate match if the claim is clear in the first step. The study design and applied methods vary depending on the classification of the claim, e.g. performance or sensory claim, consumer perception or comparative claim, ingredient-related or environmental claim. The in vivo study, for example, would be different if the claim was a general “reduces wrinkles” or a more specific “reduces wrinkles by x%” or “x test persons have stated that the product reduces wrinkles”.
In vivo studies
When the claim is set and the choice to perform an in vivo study for substantiation is made, the design of the most appropriate in vivo study includes the definition of the study panel, which should be best aligned to the intended target population of the consumer who is supposed to use the product. The investigations made should be adequate and relevant to the claim statements, they should be validated, state of the art and scientifically sound. Since human volunteers are involved, ethical considerations need to be respected so that the rights, welfare and freedom of choice for the volunteers are complied with. A further point that should be considered is the product dosage: It is best to align this with the intended instructions of use or to approximate the quantities to those used by the consumer. Methods used should be state of the art, follow guidelines where applicable, use validated, reliable and reproducible approaches. Then, when processing data and interpreting the results, the conclusions must be fair and should not overstep the limits of the study’s relevance.
Study design and choice of the right methods
From beginning to end, there is a lot to consider and all these aspects have an impact on the quality applied and achieved. As an example, the evaluation of a product’s effect on the well-being of consumer, how they feel and how they perceive themselves is a current hot topic in the cosmetic industry. With well-being, also called neurocosmetics, these products are aimed at relieving stress, adding calmness and happiness to the consumer. This is per se a very subjective approach, as every person reacts differently according to their current way of life. Therefore, it is a tough challenge for the testing industry to find appropriate study designs. Since well-being is driven by subjective reactions and emotions, the investigation should include a subjective evaluation of how the persons feel, how emotions change due to product application and how this develops afterwards. In addition, instrumental measurements can be added depending on the claim, e.g. like Electroencephalogram (EEG) measurements, Galvanic Skin Response (GSR) assessments and more. The panel should be described with corresponding inclusion and exclusion criteria, like feeling stressed in real life, showing a certain score on a generally accepted stress scale. Age and gender are typically adapted to the target population of the product. In order to receive data relevant to the topic of well-being, it is essential to closely look at the exact wording of the statement and then adjust the choice of the methods and assessment times accordingly. A typical approach is an immediate before and after application comparison, to assess the direct effect. If the long-term effect is included in the statement, the design would look very different. It includes an application phase of the product at home, documentation of sensations and emotions in a diary at home, as well as defining respective instructions for the participants. Additionally, it needs to be considered that application at home increases the variation of the data. Therefore, switching from immediate to long-term effect in the statement will have an effect on how many participants have to be included, to obtain statistically sound results.
Control treatments
Another general aspect that needs to be addressed is the inclusion of references, such as a placebo or benchmark product or an untreated reference. Typically, studies include comparisons over time and between treatments, so also the timeline investigated needs to match the claim. For example, claiming “moisturizes for 24h” requires a study period that includes the 24h assessment time and an untreated area for comparison. The untreated reference is needed in addition to the baseline measurements (before product application), so that possible effects, that come from the environment, can be excluded from the final interpretation of the results.
Human subjects and case numbers
Another point is that the participants should represent the real world, so that it should not be an ideal person, but a person from real life who would also match the intended consumer of the product. A well-prepared design includes realistic inclusion and exclusion criteria for the panel, so that the set recruitment goal and subject number can be reached in the trial. Compliance of the participants is another crucial aspect in this context. Control of their behaviour throughout the whole study period is limited. Therefore, a strong emphasis lies on giving them comprehensive instructions that should be kept during the study. Low compliance leads to higher variation of the results. Another pitfall can be the product dosage, which should be aligned with the intended instructions of use of the product. If the effect can only be seen with a dosage the consumer would not use, then this is not a valid one.
Furthermore, it has to be carefully evaluated what influences from the environment are expected and to what extent instructions help to standardize and to reduce these effects. Depending on how well instructions are expected to be kept – more likely when close to real life habits -, such variations need to be included in the consideration of panel size definition. Is, for example, a panel size of 20 sufficient and appropriate for a statement like “dermatologically tested” or do we need more participants to achieve a representative study outcome? In case of illustrative methods like LC-OCT, which gives a fantastic in vivo insight into the skin’s structural changes due to product application, sample size is often kept low for budget reasons. On the other hand, if such images shall give quantitative data by image analysis, the typical panel size of 25 to 30 participants or even more is a required number. Let us look at the following example: For evaluating the effect of newly developed anti-aging actives on collagen structure, we have implemented a new parameter to evaluate this from the LC-OCT images. First, when establishing new parameters, it is essential to validate them against well accepted ones. After validation was successful, sample size evaluation is not as simple as with more common methods. Consequently, the next step would be to perform small pilot studies to pre-screen a product’s effect. Based on these pilot studies, an appropriate sample size calculation for the claim support study can be made. With the help of this sample size calculation, the probability to find an effect as well as the final meaningfulness of the data is clearly higher than without aligning panel size in a pilot study. When experience with methods and actives is available, the panel size can be derived from known data.
Study protocol and reporting
The basis before discussing the design of in vivo studies should be a risk assessment performed by the manufacturer on what claims are intended, how specific they are and how offensive they might be to competitors in the market. Taking this evaluation into account, it is often a straightforward approach in the strategy of claim substantiation. Interpretation of the gathered results in studies, often not only one to substantiate a claim, is essential in judging if the data provide good evidence for the statement. Scientific data can be difficult to interpret, considering all the steps that should be taken. It is most helpful to define the required results for the claim in the protocol, so that the road is set for the description in the report and subsequently, for the product manufacturer on how to translate the results into a well-substantiated claim.
The study protocol includes all procedures, methods and analysis in detail. A clear objective should be described, with the relevant statistical end points to be defined. Ideally, statistical hypotheses should be given to define these in detail so that the study becomes confirmatory. This provides higher quality than exploratory approaches. All research questions related to the claim and methods should be adequately addressed, so that the study would be reproducible with this description and the outcome can be regarded as scientifically sound.
Results and outcome of the study then need a correspondingly well-composed report which describes all methods and definitions, includes protocol deviations and a scientifically based outcome. With this data at hand, it should be straightforward to relate the results to the statements and claims that can ultimately be made.
Looking at all the aspects that need consideration, a high-quality in vivo study should be based on a well-prepared study design, a detailed study protocol and truthful reporting of the results. With this in mind, misleading views, e.g. by selecting a part of the results or presenting them in exaggerated ways, should be avoided.
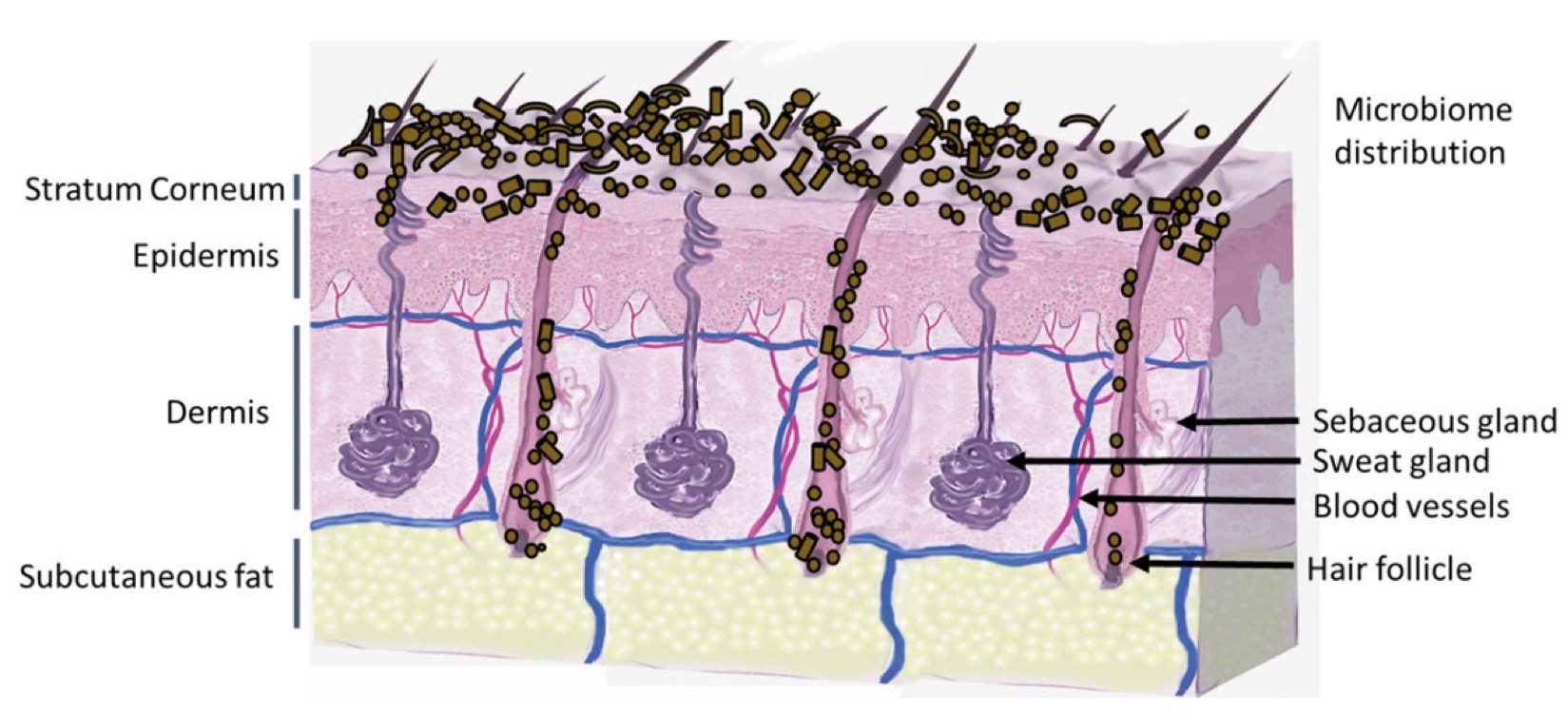
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Conclusion
Overall, in vivo studies are closer to consumer reality, compared to other possibilities, as they evaluate the consumer-product interaction. With the variety of available non-invasive methods, it is possible to achieve high credibility in the resulting outcome and thus, provide scientifically strong data to support claims for cosmetic products.

References and notes
- Arenas-Jal M, Suñé-Negre JM, Pérez-Lozano P, García-Montoya E. Trends in the food and sports nutrition industry: A review. Critical Reviews in Food Science and Nutrition. 2020;60(14):2405-21.
- Angus A. Top 10 Global Consumer Trends for 2018: Emerging Forces Shaping Consumer Behaviour: Euromonitor International; 2018 (Available from: https://tourismaccommodation.com.au/wp-content/uploads/2018/03/Top10-Global-consumer-trends-for2018.pdf.
- Labrecque LavdE, Jonas and Mathwick, Charla and Novak, Thomas and Hofacker, Charles. Consumer Power: Evolution in the Digital Age. Journal of Interactive Marketing 2013;27.
- Dunford M. Fundamentals of Sport and Exercise Nutrition 2010.
- Galaz GA. Chapter 20 - An Overview on the History of Sports Nutrition Beverages. In: Bagchi D, Nair S, Sen CK, editors. Nutrition and Enhanced Sports Performance. San Diego: Academic Press; 2013. p. 205-10.
- Bird SP. Creatine supplementation and exercise performance: a brief review. J Sports Sci Med. 2003;2(4):123-32.
- Schofield L. Vitamin Retailer The Dietary Supplement Industry Leading Magazine 2022 (Available from: https://vitaminretailer.com/activating-your-fitness-nutrition-department/.
- Newman JI, Xue H, Watanabe NM, Yan G, McLeod CM. Gaming Gone Viral: An Analysis of the Emerging Esports Narrative Economy. Communication & Sport. 2020:2167479520961036.
- Tartar JL, Kalman D, Hewlings S. A Prospective Study Evaluating the Effects of a Nutritional Supplement Intervention on Cognition, Mood States, and Mental Performance in Video Gamers. Nutrients. 2019;11(10).
