Formulation
on
Skin care
peer-reviewed
New methods of delivering ingredients that reduce the risk of microbial contamination
LAURA M. FRAZIER
Chief Scientist, TaikiUSA, Kent, OH, USA
ABSTRACT: Many consumers are looking for products to be free from preservatives, but brands want to ensure the products they are selling are safe. How can skincare products be made that satisfy both demands? One way is by developing products that have a low water activity, which reduces the risk that they will become contaminated, as microbes need water to grow. A variety of product types are being developed that fit into this category, including some newer formats such as electrospun nanofibers and freeze-dried products. This paper will investigate how lowering the water activity of a product can allow for formulations that do not contain preservatives, as well as providing an overview of the types of products that are being developed and the testing they must undergo.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
INTRODUCTION
No one wants to open their jar of face cream and see mould growing on the surface or find that their serum is home to a bacterial colony. However, many consumers, and as a result many brands, have expressed interest in using products that do not contain preservatives. In some cases, they are looking to avoid specific preservatives due to allergy concerns (1,2). Others want to avoid them altogether, either because they believe that they may be harmful to the body or they are concerned that they could have a potential negative environmental impact. Product developers are put in a difficult position. They must consider what the consumer wants, what is the least harmful to the environment, what will provide revenue for the company, what will work effectively and what is safe to use. How can they balance all of these demands?
How can a product be made safely without any preservatives, especially when cosmetic products are not stored in the refrigerator but at ambient temperature and often in a bathroom where it can get both hot and humid?
While most creams, lotions or serums have a high percentage of water and therefore need some type of preservative, there are some product types that can be formulated without preservatives and still have a low risk of microbial contamination. Primarily these are products with a high concentration of alcohol, very high or very low pH (above 10 or below 4) (3) or those with very low water activity (2). Many waterless products fall into the latter category, and these are the products that will be the focus of this paper.
The concept of water activity is extremely important in relation to the use of preservatives. While water content is the amount of water in a given sample, the water activity is a measurement of the available water, in other words, the water that is not bound and available for use (4). A product can contain water but have low water activity; honey is a classic example of this and the reason why your jar of honey will not spoil if stored properly, even though it does not contain preservatives.
The ability of microbes to grow depends on the water activity (2,5). Thus if you have a very low water activity, you will have a reduced risk of microbial contamination. So what is considered low water activity? Water activity values can range from 0 (bone dry) to 1 (the water activity (aw) of pure water or 100% relative humidity). Most microbes will not grow if aw is less than 0.7. There are a couple of exceptions, xerophilic fungi and osmophilic yeasts, which will grow between 0.6-0.7 (6,7). However, these are not the microbes typically found in cosmetics (5), so the general rule is to aim for a water activity below 0.7 (8).

Now that we have established what level of water activity is required, the next question is how to formulate in order to achieve this level of water activity? There are several ways that water activity can be reduced. One way is to remove as much water as possible. You can accomplish this through either dehydration or freezing, or a combination of both, which is known as lyophilization or freeze-drying. Adding humectants, which are materials that attract and retain moisture, can also help reduce the amount of available water. Some examples of humectants are salt, sugars, glycols, amino acids, polymers (such as hydrocolloids) or acids (2, 9, 10). There are some limitations with humectants in regards to solubility, molecular weight and toxicity. Using films, coatings or encapsulation can also assist in lowering water activity.
Within the broadly defined waterless category, there are many different product types that all have low water activity. Some of them will need to have water added just prior to using the product, whereas others are used as is. Dry mixes and powders must have water added either directly or by applying to wet skin or hair. Solid formats such as body bars can be used by simply rubbing on the skin, using body heat to melt the product enough to leave behind a layer on the skin. There are single dose options, such as those in twist-off capsules or those encapsulated in water-soluble films. Anhydrous formulations are formulated using oils or waxes, so they typically have a low water activity as a result and can be used without adding water. A recent product type that is not yet common, at least not in cosmetics, is freeze-dried products. The last category is that of nanofibres. Both freeze-dried and nanofibre products will readily dissolve with water to form a serum that can then be applied to the skin.
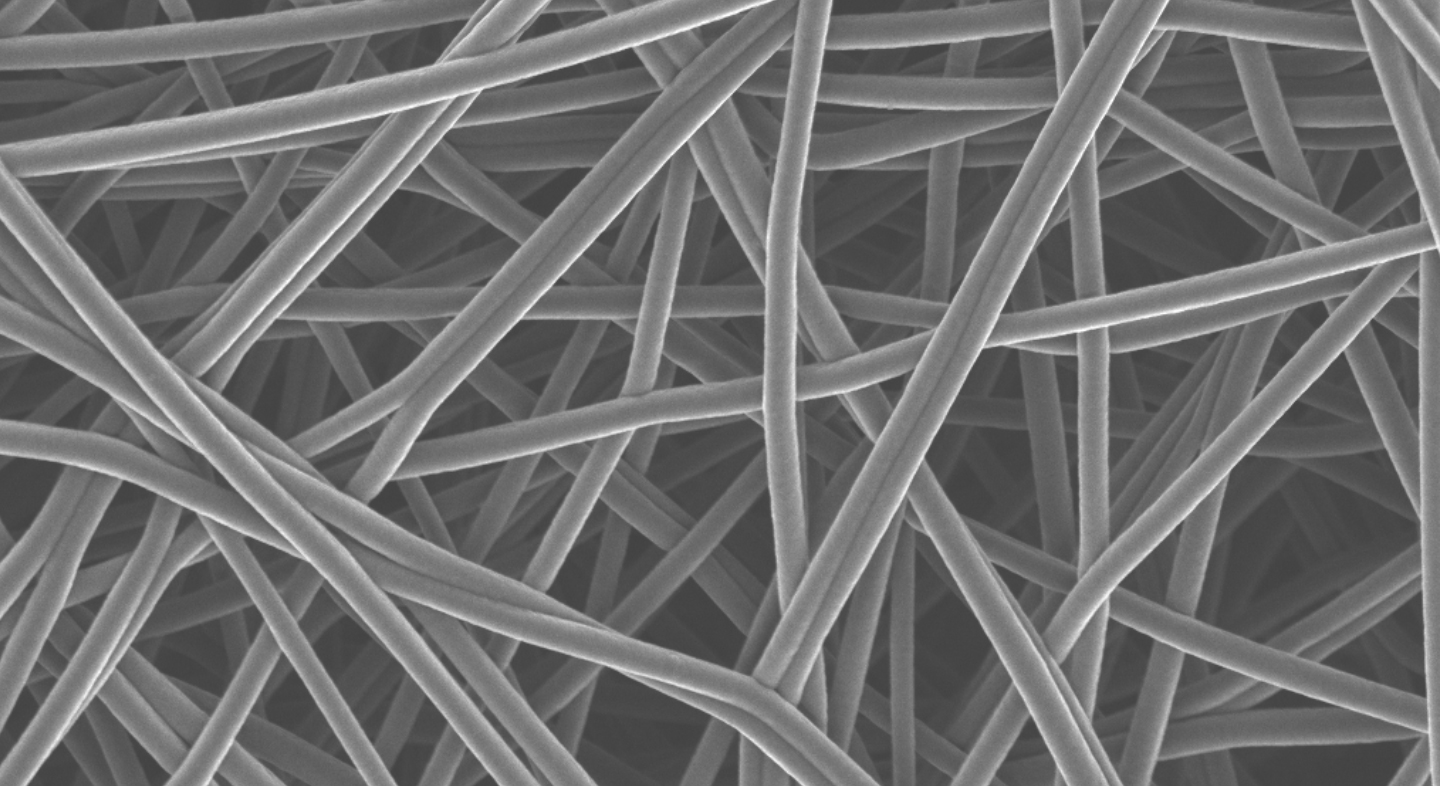
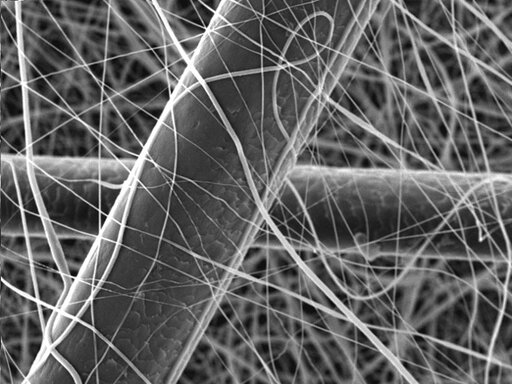
As the newest format, nanofibres are the least well known in the cosmetic space, even though they have been used for decades in other industries, such as filtration. To start off, what is a nanofibre? Well, a nanometer is 1/1,000,000,000 of a meter. Nanofibres have diameters in the nanometer range, as low as 100-1000x smaller than a human hair. The process parameters can be adjusted, however, to produce fibres with diameters above 100 nm, thus excluding them from any regulatory concerns. While nanofibres can be made using a variety of polymers, for the cosmetic industry they are primarily made from water soluble polymers such as sodium hyaluronate, polyvinylpyrrolidone, pullulan, collagen, or polyvinyl alcohol. The most common method for producing nanofibres is via electrospinning, a process that involves applying a high voltage to a polymer solution. As the solution travels through the air to the target where the nanofibres are collected, the solvent evaporates leaving behind dry fibres, which can be meters in length as the process is continuous.
Once the electrospun randomly oriented nonwoven mat is produced, it can be cut into a variety of shapes, such as undereye patches, facemasks or small circles depending on the intended use. Thin layers of nanofibres can also be stacked and moulded into different shapes, a product that is known as a nanodrop. Powders can be placed in between the nanofibre sheets where they can be protected. Moisture sensitive actives in powder form can exhibit improved stability and thereby increased efficacy by not being exposed to water at all during the manufacturing process. The water is not added until just before the customer is ready to apply. If using just the thin layer of nanofibres, the skin is simply moistened with water, and the patch is applied. The patch will dissolve immediately thanks to the high surface area to volume ratio of the nanofibres, and of course the water-soluble polymer. The nanodrop is similarly dissolved with water just prior to use, and each one is designed to be the amount needed for the specified application area, typically the face.
In order to determine if a waterless product can be safely produced without the use of preservatives, testing must be conducted, of course. Water activity testing can be conducted at relatively low cost. The easiest way is to use a water activity meter. There are different options available, which differ based on the sensor that is used (11). The resulting value has no units, as it is based on the ratio of the vapor pressure of the product compared to that of pure water.
Even if the water activity is below the aforementioned target of 0.7, testing for microbial bioburden is still necessary, as cosmetic product manufacturers are required to ensure the safety of their products per the FDA (12). Bioburden testing will identify any microbes that may have been introduced during the manufacturing process. Typically, the bioburden testing is conducted right after manufacturing as well as at the end of any stability/compatibility testing that is conducted. Poor packaging could let in moisture from the surroundings, which could alter the water activity of the product and enable microbial growth. The final product should always undergo shelf-life testing and accelerated aging in the final packaging in order to ensure that there are no such changes to the product over time.
Beyond the benefit of not requiring a preservative, waterless products have other positive attributes as well. They can be convenient and travel friendly, given they are in solid form and not liquid. In some cases, they can be packaged using more sustainable materials and less material overall. Some even have the potential to be more affordable. Quite possibly the most important aspect is the ability to increase the stability of certain active ingredients, particularly those that are known to degrade when exposed to aqueous environments. The product is typically lighter as well, since the water has been removed, which could reduce the energy required for shipping.
So should you try to formulate your products without preservatives? As is almost always the case with scientific topics, the answer is much more nuanced than a simple ‘yes’ or ‘no’. It depends on who your target customer is. It depends on what ingredients you want to include in the formulation. It depends on how the product will be applied. It depends on the price point of the final product. It just…depends.

Human hair covered in nanofibres
Optical microscope
Scanning electron microscope SEM)
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
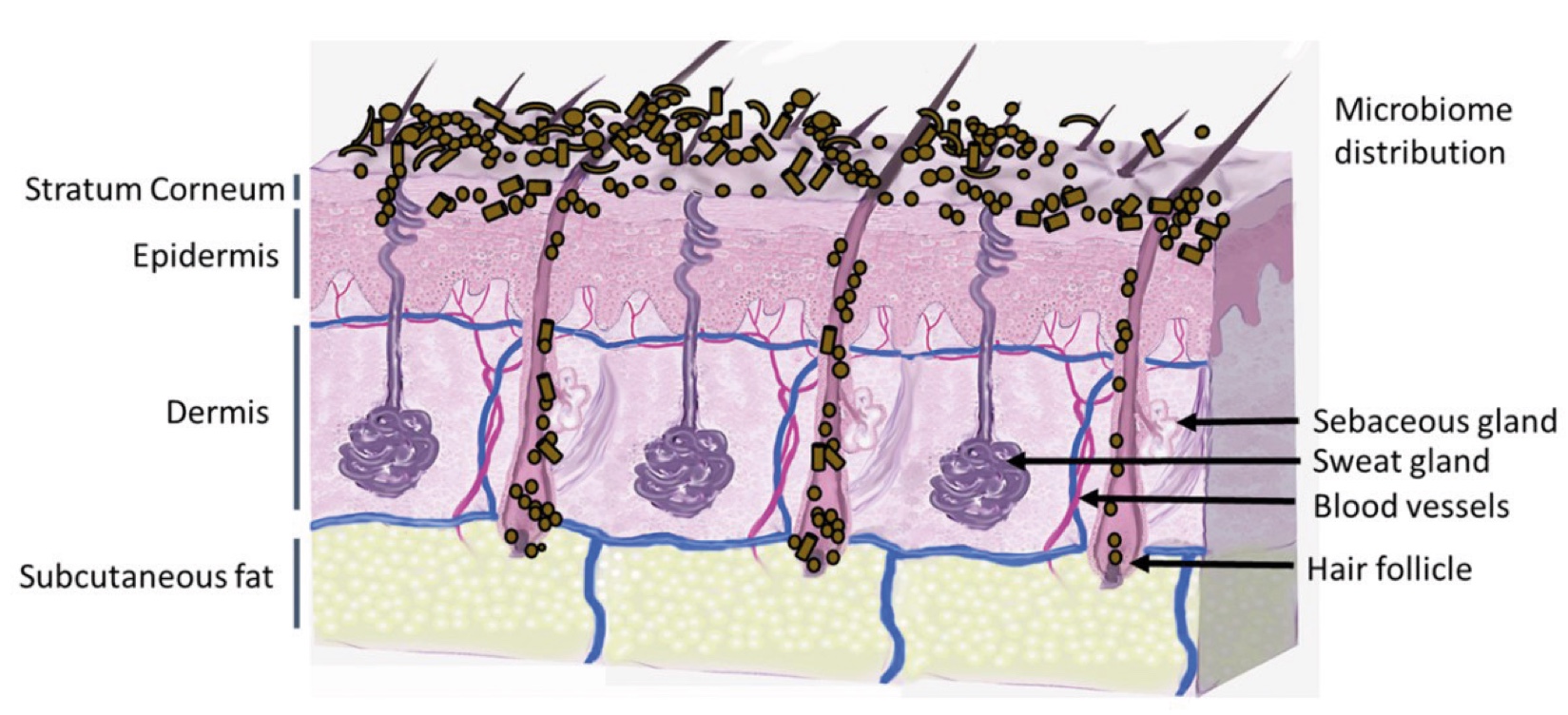
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Lundov et al. Contamination versus preservation of cosmetics: a review on legislation, usage, infections, and contact allergy. Contact Dermatitis. 2009; 60: 70–78 (https://pubmed.ncbi.nlm.nih.gov/19207376/)
- Varvaresou A, Papageorgiou S, Tsirivas E, Protopapa E, Kintziou H, Kefala V, Demetzos C. Self-preserving cosmetics. Int J Cosmet Sci. 2009; 31(3):163-75. doi: 10.1111/j.1468-2494.2009.00492.x. Epub 2009 Mar 19. PMID: 19302511. https://pubmed.ncbi.nlm.nih.gov/19302511/
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018; 23:1571. https://doi.org/10.3390/molecules23071571
- https://www.gov.mb.ca/agriculture/food-safety/at-the-food-processor/water-content-water-activity.html
- Philip A Geis, PhD. 2021. Cosmetic Microbiology A Practical Approach. 3rd Edition. Abingdon, England: CRC Press.
- https://www.arlok.com/news/Water-Activity
- https://pmp.errc.ars.usda.gov/wateractivity.aspx
- https://www.cosmeticsandtoiletries.com/research/methods-tools/article/21836223/water-activity
- Nadarzynski, A.; Scholz, J.; Schröder, M.S. Skin Barrier Enhancing Alternative Preservation Strategy of O/W Emulsions by Water Activity Reduction with Natural Multifunctional Ingredients. Cosmetics2022; 9(3): 53. https://www.mdpi.com/2079-9284/9/3/53
- Kerdudo, A., Fontaine-Vive, F., Dingas, A., Faure, C. and Fernandez, X. Optimization of cosmetic preservation: water activity reduction. Int J Cosmet Sci. 2015;37: 31-40. https://doi.org/10.1111/ics.12164
- https://foodindustryexecutive.com/2023/07/beyond-food-safety-the-many-ways-water-activity-impacts-food-processing-and-packaging-qa-with-mary-galloway-of-meter-food/
- https://www.fda.gov/cosmetics/potential-contaminants-cosmetics/microbiological-safety-and-cosmetics
