
Microbiome
on
Skin care
peer-reviewed
Is Microbiome Certification even Necessary?
ALBERT DASHI, PHD1, PETRONILLE HOUDART, DPHARM2, OLIVER WORSLEY, PHD3
- CSO and Co-Founder at Sequential Skin Ltd
- Skincare Director and Co-Founder at Sequential Skin Ltd
- CEO and Co-Founder at Sequential Skin Ltd
ABSTRACT: This article explores the intricate regulatory landscape of the beauty and personal care industry, characterized by a delicate balancing act between consumer expectations and scientific advancements in product formulations. With a rise in certifications aiming to establish industry standards grounded in science, the absence of robust global regulations has led to diverse methodologies lacking clear effectiveness criteria. As brands and formulators take greater responsibility for their products, the demand for higher testing standards emerges. Delving into this dynamic, the article highlights the need to discern credible certifications and identifies testing protocols poised to withstand future regulatory scrutiny, shedding light on key considerations shaping the industry's trajectory.
??????????????????
Introduction
When it comes to understanding the regulatory landscape of the beauty and personal care industry, we often find ourselves standing on a tightrope with little guidance on where to go, balancing consumer’s expectations as well as newfound science upon which formulations are developed. In recent years, we have seen a spike in companies bringing out microbiome certifications under the guise of instilling industry norms rooted in science.
Unfortunately, due to lack of stringent regulations from the world’s governments, the field is left open for every methodology, with little guidance on which protocols are bringing a truly effective service that will draw meaningful conclusions rooted in quantifiable data. As brands and formulators begin to take accountability for the products they are bringing forward to the industry, it is crucial to hold testing companies to a higher standard.
In this article, we will review why the microbiome has gained such traction, what theories are being applied to the development of products that target it, and what testing protocols are currently being seen within the industry and their merits when it comes to claims and certifications.
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
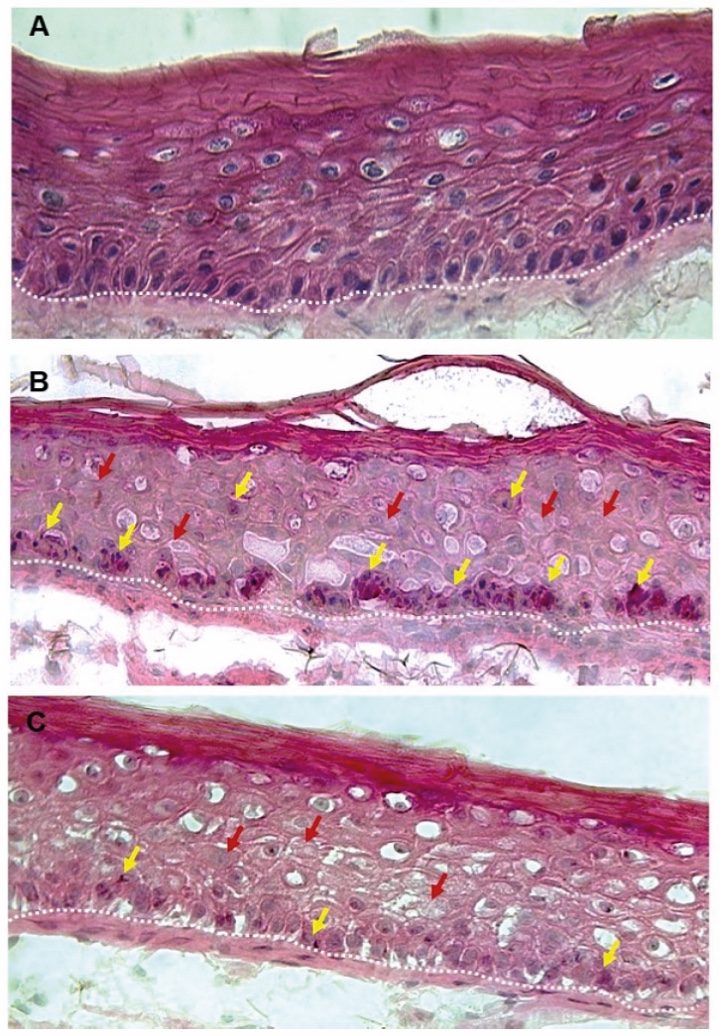
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
WHY HAS THE SKIN MICROBIOME GAINED SUCH TRACTION WITHIN THE PERSONAL CARE INDUSTRY?
The skin microbiome consists of an organic ecosystem of trillions of bacteria, fungi and other microorganisms that act as a natural barrier, interacting with one another, defending against potential pathogens and contributing to the skin’s overall health and resilience (1). [GC1] The microbiome of every individual is entirely unique, shaped by their external environments as well as the substances they are exposing their skin to. The microbiome can be impacted by factors such as stress, pollution, personal care products and even the countries within which we reside.
Due to increased knowledge on the microbiome, respecting its delicate balance has become paramount in formulating effective and innovative personal care products. It is known that when the skin microbiome is in dysbiosis (when microbial diversity is imbalanced), it can lead to skin conditions such as rosacea, acne, atopic dermatitis, and many others (2).
The microbiome has become a central consideration in the development of skincare solutions, representing a transformative shift towards products that not only enhance the appearance of skin but also contribute to the overall health and well-being of it (3). The vast majority of key players in the industry have understood that the microbiome is not just a trend that will dissolve in a few months or years, it is an important component of medicine, which will impact every field of human health, and relating to personal care.
CERTIFICATION: LANGUAGE AND MARKETING AROUND THE MICROBIOME
A significant portion of the discourse surrounding the question of whether microbiome certification is even necessary stems from the wording of the certifications themselves. The industry has begun to adopt terms such as “microbiome-friendly” and “biome friendly,” without full research being carried out in understanding these human-related microbes, using sophisticated technology such as Next Generation Sequencing.
The scientific community that is driving the research within the area are beginning to express the view that these words hold little meaning and inaccurately represent the complexity of what is occurring in the microbiome. On the other hand, industry thought leaders are seeking to simply educate their consumers to lower the barriers to entry. Both the science and the education of consumers is imperative for the microbiome movement to make headways, but the varying approaches to the science and its explanation has resulted in certifications of different calibre.
There are those who are marketing their products as having microbiome benefiting results without doing any testing, highlighting ingredients that have been found to balance the microbiome. Unfortunately, due to lack of proper data to support their claims, such brands can find themselves under major scrutiny, especially in the North American market where false claims can result in legal repercussions as was seen with a large FMCG’s portfolio company in 2021. The best solution to this problem is to pick the correct testing to support claims.
TYPES OF MICROBIOME TESTING AVAILABLE IN THE INDUSTRY
In the realm of scientific research and product development, the importance of testing methodologies cannot be overstated. When it comes to understanding the intricate world of bacteria and microbes, there are numerous testing methodologies that are currently being adopted by the industry: in-vitro, ex-vivo, and in-vivo.
in vitro Microbiome Testing: is conducted in a controlled, artificial environment such as a lab and typically focuses on isolated bacterial strains to assess interactions between the product and the isolated bacteria. Although it can allow for precise examination of how a bacteria responds to products, it neglects to take into account the complex and dynamic nature of the microbiome as a whole. This form of testing can be crucial within the context of medicinal research, where it might be crucial to understand the relationship between a treatment product and a pathogenic bacteria that could not otherwise be tested safely in human subjects. It can also serve well for more upstream analysis, when working with raw ingredients that are still undergoing exploration before they are infused into formulations. However, when it comes to the microbiome, there is still little evidence to support that in vitro approaches carry sufficient data to back microbiome claims in human.
ex vivo Microbiome Testing: involves the study of the microbiome outside of its natural living context by using skin samples and intact tissue. This enables the examination of microbiome responses to products in a controlled yet biologically relevant setting. It is viewed as a middle ground between in vitro and in vivo. The limitation are often: access to tissue; batch effect with the tissue of origin; difficulty in co-culturing methologidies (the same microbes we’ve traditionally tried to remove from cell culture with antibiotics!); and finally, difficulty in longer term viability in culture, for longitudinal studies on the skin.
In vivo Microbiome Testing: is conducted directly on human skin, providing insights into the behaviour of the microbes in their organic environment and offering a thorough understanding of microbial interactions. This type of testing allows for a proper assessment of a product's impact on the microbial balance and diversity as well, something that cannot be accomplished with other forms of testing. However, as with all methodologies, there are a few limitations when it comes to the in vivo approach. For starters, there is still much research to be carried out in order to fully characterise the multitude of strains, and more importantly, the interaction of these microbes with themselves – and us, their host. There are also debates concerning the extraction of microbes and how to determine whether they are dead or live upon collection and what this means functionally. There is considerable R&D that is being pushed on this side to minimise the ambiguity through the use of qPCR approaches for both DNA (what’s there) and RNA (what is active), henceforth, there is still much to uncover but the in vivo method still provides the best confidence of what is occurring in human.

HUMAN IN VIVO MICROBIOME TESTING: A HOLISTIC METHODOLOGY
Despite the numerous certifications gaining traction within the industry, the key to understanding whether or not it holds any weight and value stems from what hypothesis it is trying to test. In order for a product to be tested and certified with any confidence, the answer to the following four questions are crucial:
- Is there any significant change in the total microbial diversity before and after product usage, longitudinally?
- Does the product alter the balance of commensal to opportunistic microbes?
- Are there predictive functional and metabolic changes, from the microbiome profile, that are maintained or changed after product usage?
- Can we associate any significant biophysical changes (e.g. pH, hydration, elasticity) or meta-data (e.g. dermatology assessments, treatment, imaging, age, ethnicity) with specific microbial changes?
Unfortunately, unless a product is evaluated on live human skin, these answers cannot be discovered, nor have in vitro tests been reliably validated against in vivo or clinical microbiome tests. The total diversity of the skin microbiome can only be measured when taking a sample from human skin and quantifying it. Unlike in vitro and ex vivo models of testing, the in vivo approach offers relevant data that can be extrapolated to the general population and translated into sound claims.
Studying Impact on Atopic Skin
When testing on human subjects, there is a huge opportunity to test products formulated for specific skin conditions to see how they will interact with those individuals' unique microbiome. The personal care industry is often driven by the need to problem solve consumer concerns, creating formulations that will target specific niches. If a brand is looking to test a product designed especially for acne-prone skin, recruiting individuals with this skin concern is the only logical step in uncovering whether or not their product will help their condition and support their microbiome. These are considerations that are overlooked when going the in vitro route. Indeed, in studying conditions like atopic dermatitis, there have been key strains involved in the inflammatory process, and likelihood of flareups. Something that requires strain-level analysis in vivo to uncover.
Long-term Effects of Formulations
in vivo Human testing allows for longitudinal analysis (evaluating usage of the product over time), giving deeper insights into a product's impact on the microbiome rather than a brief snapshot of how the product interacts with an isolated bacteria. A well structured study should be centred around multiple sample collection points, giving significant time to pass between collections. This allows brands to review how a product is performing before and after usage, but also take into account its gradual impact on the microbiome.
Measuring Products Against Control Groups
Human testing provides an additional advantage by allowing the evaluation of formulations containing active ingredients in comparison to control groups. This expands the breadth of the study, illustrating the effects of an active ingredient or its concentration in a product when contrasted with a group that is exposed to a basic formula. This approach enables a more comprehensive understanding of the formulation's influence on microbial balance and diversity.
Expanding the Scope of the Study
Although the microbiome is gaining traction on the research and development side of the industry, the consumers of skincare and personal care products are still seeking solutions to their primary concerns: acne, rosacea, atopic dermatitis, skin elasticity loss, etc. This is why a human approach to testing the microbiome brings forward the opportunity to evaluate it against other metrics, such as biophysical aspects such as skin hydration, elasticity, pH, etc. Unlike isolated lab testing, in vivo microbiome testing can be used for correlation analysis between other areas and the microbiome.
CERTIFICATION ASSOCIATED COSTS
The costs associated with microbiome testing for consumer personal care products is an important one to take into account. In vitro testing stands as the more cost-effective approach and limits the factors needed to take into consideration when setting up a study. However, it is important to realise that this level of testing and the certification associated with it might not be robust enough when regulations are put into place where the microbiome is concerned.
On the other hand, the in vivo approach can lean on the pricier side, there is significant research to support the claims being put in place and the certification that accompanies it. With the need to test on human subjects, recruitment and analysing the microbiome against other biophysical elements, the costs associated rival clinical studies with the promise of relevant data that will hold value over time.
REGULATIONS IN THE PERSONAL CARE INDUSTRY
The personal care industry is currently grappling with a notable absence of comprehensive regulations, leaving a void in oversight and accountability. This lack of framework has raised concerns regarding the consistency and reliability of product claims, potentially exposing consumers to uncertainties about the efficacy and safety of microbiome-based formulations (4). Without standardised guidelines, there will remain a risk of misleading marketing practices, allowing for the proliferation of products that may lack scientific validation or adhere to established quality standards. The absence of regulation poses a challenge not only in ensuring consumer protection but also in fostering a trustworthy and transparent marketplace for microbiome-related personal care products. As the industry continues to evolve, addressing these regulatory gaps is becoming essential to safeguard both consumer interests and the integrity of the products entering the market.
In conclusion, the necessity for regulations in the microbiome certification of personal care products remains an important discussion. However, amidst the ongoing need for regulatory frameworks, there exists an opportunity for brands to make informed choices. Armed with a better understanding of the essential components that constitute a robust study, brands can select certifications that align with the academic calibre of clinical studies. This not only enhances the credibility of their marketing claims but also contributes to the establishment of industry standards that prioritise both consumer well-being and scientific rigour in the evolving landscape of microbiome-focused personal care.

Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
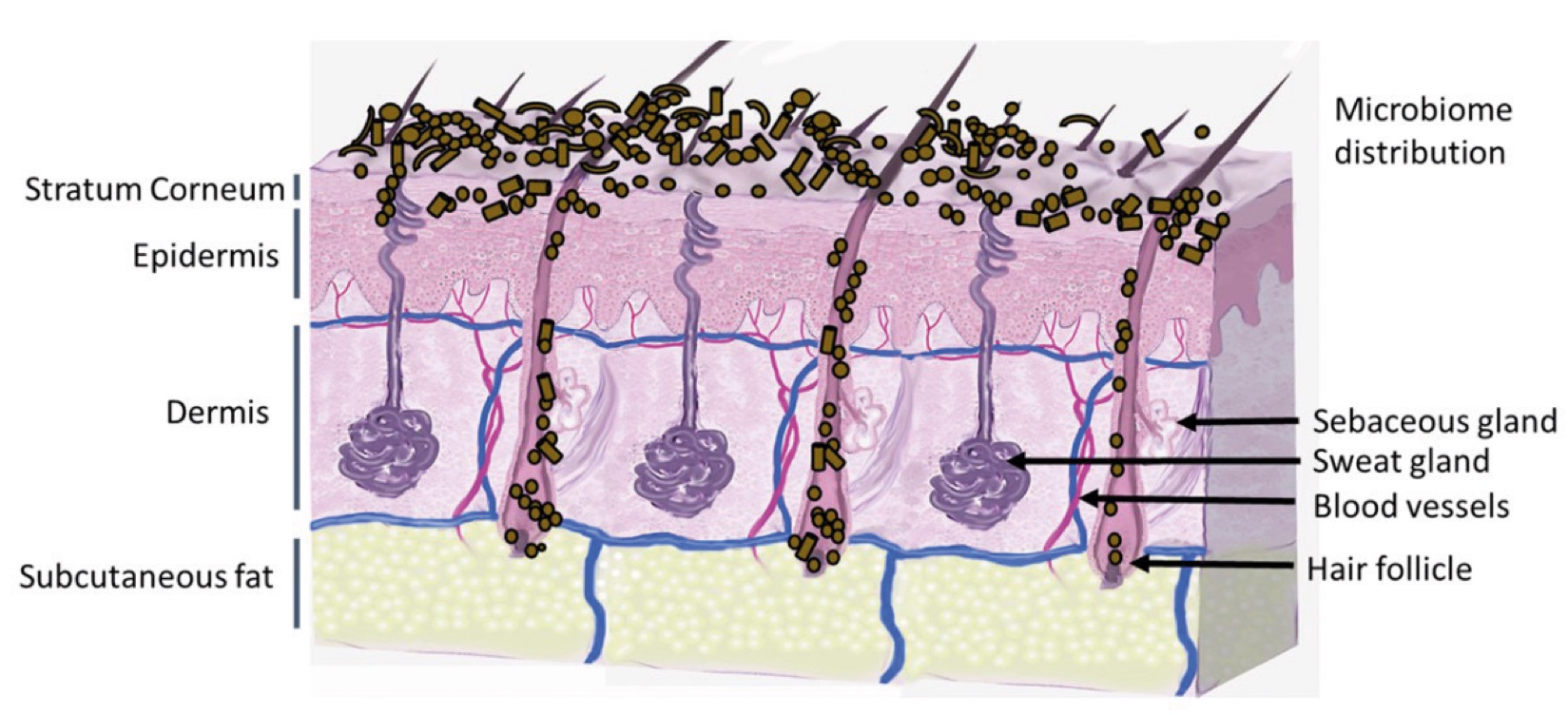
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Berg, G., Rybakova, D., Fischer, D. et al. (2020) Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103. https://doi.org/10.1186/s40168-020-00875-0
- Lee HJ, Kim M. (2022) Skin Barrier Function and the Microbiome. Int J Mol Sci. 28;23(21):13071. doi: 10.3390/ijms232113071. PMID: 36361857; PMCID: PMC9654002. https://www.mdpi.com/1422-0067/23/21/13071
- Akim, T. (2022) Beyond the gut: Here’s why microbiome skincare is the next big thing, Byrdie. Available at: https://www.byrdie.com/microbiome-skincare-4783223 (Accessed: 02 February 2024). [GC1]
- Santoni O. (2021) Microbiome Claims: Should Pre-, Pro- and Postbiotic Skin Care Be Regulated?Cosmetics & Toiletries. Available at: https://www.cosmeticsandtoiletries.com/regulations/claims-labeling/article/21836088/cosmetics-toiletries-magazine-microbiome-claims-should-pre-pro-and-postbiotic-skin-care-be-regulated (Accessed: 02 February 2024).


