Cyclization as a tool
for reinvention
ARIADNA GRAU-CAMPISTANY*, PATRICIA CARULLA, LAIA FARRÉ, SILVIA PASTOR
*Corresponding author
Lipotrue, Barcelona, Spain
ABSTRACT: Due to the favourable characteristic of cyclic peptides, such as the higher stability and lower toxicity, they are attractive candidates for the development of therapeutics. Protein misfolding increases with age, and the accumulation of these misfolded or unfolded proteins causes ER stress. An existing linear peptide was cyclized in order to find a novel active ingredient, which was able to act on this ER stress by improving the UPR efficiency while boosting collagen. Overall, improving the aging skin and balancing the fluctuation between morning and evening wrinkles.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
INTRODUCTION
Natural and synthetic peptides are widely used in the cosmetic industry, mostly as antiaging products due to their high activity and low toxicity. Linear peptides have been broadly developed with success in the cosmetic industry for more than 20 years (1). Cyclization comes as a new approach to enhance stability against enzymatic and chemical degradation. Moreover, it can be used to synthesize analogues that mimic the biological activity of natural structures while presenting increased permeation, enhanced bioavailability, reduced conformational flexibility that often leads to a better interaction with its target and hence an improved biological activity (2). Nevertheless, cyclic peptides are still quite unknown in the cosmetic industry despite the many advantages they offer.
Protein structure is of outmost importance as it is linked to protein function. Protein folding is the process by which a polypeptide chain folds to become a biologically active protein in its 3D structure and takes place in the endoplasmic reticulum (ER). Under normal circumstances, proteins are translated into the ER via ribosomes embedded in the ER membranes, bound by chaperone proteins, folded and then packaged into vesicles for secretion. In some cases, proteins can become misfolded or unfolded in the ER, and this protein is then targeted for degradation by the proteasome. However, this process is not always perfect, and environmental, cellular or molecular factors can prevent the proper turnover of misfolded or unfolded proteins, leading to their accumulation and aggregation. This causes a cellular condition known as ER stress (3). ER stress is associated with loss of protein function and cell death. To avoid this, the cell resolves misfolded protein stress via the unfolded protein response (UPR) which takes place in the ER (4). This protein quality control mechanism is essential for maintaining the function and integrity of cellular processes. However, external influences including aging, cause the UPR itself to become impaired, which leads to further accumulation of misfolded proteins which in turn causes further UPR impairment.
In mammals, the UPR is formed by three branches or ER stress sensors: the inositol requiring kinase 1 (IRE1), double stranded RNA-activated protein kinase-like ER kinase (PERK) and the activating transcription factor 6 (ATF6) (4). When the UPR is activated, ER chaperone proteins (such as BIP and Calreticulin) dissociate from these sensors, allowing their activation which in turn activates downstream pathways. However, during aging, the function of the UPR is compromised, since there is a progressive failure of the chaperoning systems and a decline in the UPR components, so the UPR activation cannot rescue the ER stress (5).
We show here, how by means of cyclizing an existing linear peptide, we have obtained a totally novel molecule, peptide c[E5Ch-H] (INCI: cyclohexapeptide-90), with a completely new mechanism of action aiming to target the ER response.
MATERIALS AND METHODS
Peptide synthesis
The peptide was obtained by using standard solid phase Fmoc protocols (6). Cyclization was performed in solution. The crude peptide was purified using a high-pressure liquid chromatography (HPLC) device from Shimadzu (Kyoto, Japan) on a preparative Waters C18 column using a water/acetonitrile gradient supplemented with 0.1% TFA. The identity of the purified peptide was confirmed by ESI mass spectrometry, and analytical HPLC showed it to be at least 95% pure.
Calreticulin Immunofluorescence
Human Epidermal Keratinocytes (HEKa) were cultured on coverslips in Dermal Cell Basal Medium (DCBM) at a density of 50.000 cell/well and incubated at 37°C in a humidified atmosphere with 5% CO2. When cell confluence was around 80%, cells were treated with c[E5Ch-H] at 0.005 mg/mL for 24 h. After treatment, samples were washed with PBS, fixed with 4% formaldehyde, permeabilized with Triton X-100 0.2% and blocked for 1 hour with PBS/BSA 5%.
For CALR staining, samples were incubated for 90 min with Calreticulin Polyclonal antibody (1:500 in PBS/BSA 1%, RT). After washing with PBS, cells were incubated with 5 μg/mL of the secondary antibody Goat anti-Rabbit IgG Alexa Fluor 488 (1 hour, RT). 0.4X Phalloidin-TRITC was added to stain actin filaments. Finally, samples were washed with PBS, mounted using Prolong-Gold with DAPI (nuclei staining) and kept protected from light at 4ºC.
Microscopic images were acquired (40x). At least five different fields from each coverslip (three per condition) were acquired using the same settings.
Ex vivo assay
Skin explants were obtained with the informed consent from abdominal surgery from a female donor (42 y/o, phototype III) and kept in survival in BEM culture medium (BIO-EC’s Explants Medium) at 37°C in a humid, 5% CO2 atmosphere.
A cream containing 1% of c[E5Ch-H] was topically applied (2 mg/cm2) on day 0, 2, 5 and 7. Brefeldin-A (0.15 μg/mL) was added to the culture medium on D5, to induce endoplasmic reticulum stress with accumulation of unfolded or misfolded proteins. The control explants did not receive any treatment except the renewal of culture medium. The culture medium was half renewed (1 mL/well) on D1 and D2 and fully renewed (2 mL/well) on day D5 (brefeldin-A containing medium) and D6 (brefeldin-A free medium).
Explants were then collected and fixed in buffered formalin solution for 24 h before being dehydrated and impregnated in paraffin using a Leica PEARL dehydration automat. The samples were embedded using a Leica EG 1160 embedding station. 5-μm-thick sections were made using a Leica RM 2125 Minot-type microtome, and the sections were mounted on Superfrost® histological glass slides. The microscopical observations were realized using a Leica DMLB, an Olympus BX43 or BX63 microscope using cellSens storing software (Olympus).
Immunostaining was performed using 1) a polyclonal anti-Calreticulin antibody (Invitrogen, ref. PA3-900; 1:3000 in PBS-BSA 0.3%-Tween 20 at 0.05%) and 2) a polyclonal anti-CHOP antibody (Novus Biologicals, ref. NB600-1335; 1:200 in PBS-BSA 0.3%-Tween 20 at 0.05%). Both were incubated for 1 h at RT using a Vectastain Kit Vector amplifier system avidin/biotin, and revealed by VIP (Vector laboratories, Ref. SK-4600). The immunostaining was assessed by microscopical observation.
Keratinocytes-Fibroblasts communication assay
For RNA extraction, HEKa were seeded in duplicate in 12-well plates (150.000 cells/well) in DCBM and incubated at 37°C in a humidified atmosphere with 5% CO2. Next, HEKa were incubated with 0.0025, 0.005 or 0.01 mg/mL c[E5Ch-H] during 48 h more.
In this moment, HDF were seeded at 200.000 cells/well in 106 Medium (M106500, ThermoFischer Scientific) with 2% LSGS and incubated for 24 h at 3°C in a humidified atmosphere with 5% CO2. Then, cells were deprived for 24 h with medium 106 with 0.1% LSGS.
After 48 h treatment with the candidate, conditioned medium from HEKa was collected and transferred to deprived HDF and incubated for 24 h more. HDF cells were lysed and pooled together, and gene expression was measured.
For immunofluorescence staining, HEKa were seeded in triplicates in 24-well plates at 100.000 cells/well in DCBM. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2. Next, HEKa were incubated with 0.0025, 0.005 or 0.01 mg/mL c[E5Ch-H] during 48 h more.
In this moment, HDF were seeded on coverslips at 50.000 cells/well in 106 Medium with 2% LSGS and incubated for 24 h at 37°C in a humidified atmosphere with 5% CO2. Then, cells were deprived for 24 h with medium 106 with 0.1% LSGS.
After 48 h treatment with the candidate, conditioned medium from HEKa was collected and transferred to deprived HDF and incubated for 4 days more.
After treatment, cells were fixed with 4% formaldehyde, washed with PBS and finally permeabilized and blocked for 1 hour with PBS-Triton 0.2%X-100/BSA 5%. For collagen I staining, samples were incubated 2 hours at RT with Collagen I antibody diluted 1:100 in PBS-Triton 0.2%X-100/BSA 1%. After proper washing, cells were incubated with 5 μg/mL of the secondary antibody Goat anti-Mouse IgG Alexa Fluor 488 (recognition of primary antibody), during 2 hours at RT. Finally, microscopic analysis was performed as explained above.
Clinical assay
Clinical efficacy was assessed with 20 Caucasian female volunteers (40-60 y/o) with clinical signs of aging. Efficacy was determined versus placebo, half-face, for 28 days with a twice a day application regime and measurements performed twice a day, in the morning (8.30-10.30 am) and in the evening (4.30-6.30 pm). Evaluations were carried out by means of non-invasive bioengineering techniques able to measure skin profilometric parameters: wrinkles depth and volume in crow’s feet area (Primos 3D), and skin thickness in cheeks (ultrasound DUB® SkinScanner).
Statistical analysis
For in vitro assays, each condition was performed at least in triplicate (n=3) in three independent experiments (N=3). All data was normalized versus basal except for cell death assays where it was normalized vs BFA. Statistical data analysis was performed using Student t-test by comparison of % of response from test substance vs response of the damage or untreated control condition wells (Basal) where ***p<0.001, **p<0.01 and *p<0.05. All graphics show the mean ± SEM. For the clinical assay: ***p<0.001 vs T0, **p<0.01 vs T0, *p<0.05 vs T0 and ***p<0.001 vs placebo.
RESULTS AND DISCUSSION
The cyclic peptide increases ER proteins
Different factors can disrupt the ER homeostasis resulting in an accumulation of misfolded and unfolded proteins, a condition known as ER stress activating the Unfolded Protein Response (UPR) (4). However, if the UPR fails to reestablish the ER, ER stress causes cell dysfunction and death. Interestingly, aging cells present decreased number of ER proteins (e.g. BiP) and the limited chaperones appear to be impaired, also altering the threshold at which the UPR switches to the apoptotic pathway (4). Skin, especially aging skin is also affected by this protein misfolding, making it appear more tired and wrinkled. Therefore, finding an active ingredient able to act on protein misfolding is of high interest in the cosmetic industry.
By means of cyclizing an existing and proprietary linear peptide, which was designed in silico to act on the neuromuscular junction and has shown clinical efficacy against expression wrinkles, we have obtained a totally novel molecule with a completely new mechanism of action aiming to target the ER response. This cyclic peptide, c[E5Ch-H], is able to increase Calreticulin, firstly observed by transcriptomic analysis and further confirmed by qPCR (data not shown). This increase in CALR has not been observed for the linear counterpart.
This activity was additionally investigated by analyzing Calreticulin protein levels by immunofluorescence. After a 24 h-treatment with 0.005 mg/mL c[E5Ch-H], this compound is able to significantly enhance the expression of CALR protein by 79%***, when compared with untreated cells (Figure 1, A-B).
Moreover, calreticulin increase was also confirmed ex vivo. Skin explants were treated with a cream containing 1% of c[E5Ch-H] and Calreticulin was measured by immunostaining. The cyclic peptide caused a significant increase of 52%** in calreticulin (Figure 1, C-D), which further verifies what was previously observed at the gene and protein level.
Calreticulin is a highly conserved ER chaperone protein that identifies N-linked glycoproteins and ensures proper protein conformation and prevention of protein aggregation. The peptide is also able to increase BiP (data not shown), the most important chaperone in the UPR, which binds to misfolded proteins and folds them properly. This is of outmost importance, because as we age, BiP levels decrease and therefore, the UPR activation cannot rescue the ER stress.
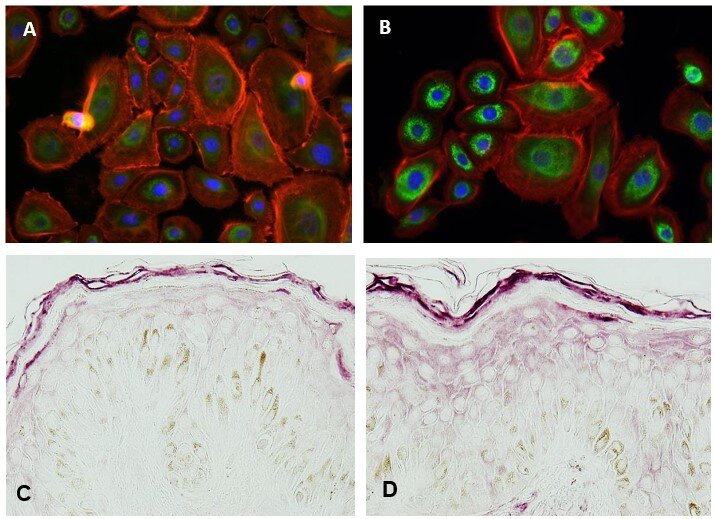
Figure 1. Representative images for CALR expression: (A) Non-treated HEK and (B) HEK after 24 h treatment with 0.005 mg/mL c[E5Ch-H], acquired with a fluorescence microscope (green: CALR, blue: nuclei, red: phalloidin). (C) Non-treated skin explants and (D) Skin explants after treatment with a cream containing 1% of c[E5Ch-H], acquired using microscopy (violet: CALR).
The cyclic peptide acts on ER stress
As previously mentioned, when cells undergo an irreversible ER stress that overcomes UPR capacity, this pathway eliminates damaged cells by apoptosis (7). Importantly, during aging, there is a shift in the balance between the protective adaptive response of the UPR and the pro-apoptotic signaling, where the protective response is significantly reduced, and the apoptotic response is more robust. Therefore, we evaluated if the peptide was able to protect from cell death. To do so, caspase-3 was evaluated by induction of ER stress using Brefeldin A. The peptide was successful in significantly decreasing caspase-3 (-17%***), which was increased by BFA incubation (+50%***). Similar results were obtained in an ex vivo assay, where BFA was also used to induce ER stress. In this case CHOP was measured. CHOP, also known as growth arrest and DNA damage 153 (GADD 153) is a pro-apoptotic protein, which has been shown to increase with stress during aging (5). The authors have not been able to find in the literature any reference of protein misfolding assays in skin explants.
Epidermis & dermis communication due to c[E5Ch-H]
Moreover, CALR has been shown to present non-endoplasmic reticulum functions such as the regulation of the production of the ECM (8). Collagen expression appears to be regulated by ER calcium levels and intracellular CALR. In addition, CALR has indirect effects on collagen matrix deposition as a consequence of its role in regulation of fibronectin expression (9) as well as the increase of TGFβ3 and integrins β1 and α5 (10). Because the peptide was able to increase calreticulin in keratinocytes we wanted to see the indirect effect on fibroblasts. It is known that keratinocytes participate in the maintenance of skin homeostasis by actively cross talking with fibroblasts. In particular, the cytokine Transforming growth factor-β (TGF-β) is considered a master regulator of ECM induction (e.g. collagen I, elastin and fibronectin) and deposition through the activation on Smads signaling pathway (11). The isoform TGFB3 has been shown to increase collagen transcription and synthesis (12) and more specifically, TGF-β3 is believed to regulate molecules involved in cellular adhesion and extracellular matrix (ECM) formation in response to extracellular calreticulin (eCRT). Therefore, we evaluated how the treatment of human epidermal keratinocytes with c[E5Ch-H] modulates TGFβ3 gene expression and collagen I protein levels in human dermal fibroblasts. We could see how TGFB3 was increased (1.60-fold) in fibroblasts when treated with keratinocyte conditioned media (HEK treated with 0.01 mg/mL cyclic peptide) and more importantly, collagen was very significantly increased, therefore confirming that the peptide has a clear anti-aging efficacy by boosting the ECM (Figure 2).
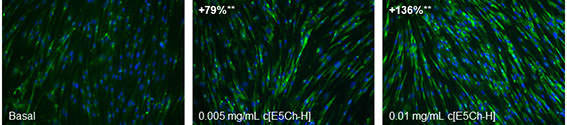
Figure 2 Collagen I expression in HDF cells after 4 days of treatment with conditioned media from untreated (Basal) or c[E5Ch-H]-treated HEKa. Representative images acquired with a fluorescence microscope (green: Collagen I, blue: nuclei).
The cyclic peptide shows anti-aging efficacy and improves the fluctuation in the wrinkles daily cycle
Finally, clinical efficacy was assessed on volunteers with already existing aging signs. Wrinkle depth and volume was measured in crow’s feet with Primos 3D while skin thickness in cheeks was measured using an ultrasound. More importantly, measurements were performed in the morning and in the evening, because it has been described that wrinkles are more pronounced in the afternoon versus the morning (13) while skin is thinner in the afternoon (14) due to dermal fluid redistribution as swelling tends to occur in the morning due to the effects of gravity during sleep and wrinkles may be swollen in the morning while repeated movements of the face due to changes of facial expression may gradually increase wrinkle formation and depth from the morning to the afternoon. It was very interesting to confirm what was described in the literature, as we could also observe in the volunteers how they had a thinner and more wrinkled skin in the evening. A decrease in wrinkle depth and volume in crow’s feet was achieved with application of a cream containing 3% of c[E5Ch-H], but most importantly, the fluctuation between morning and evening wrinkles was decreased. Similar results were obtained for wrinkle volume (data not shown). Moreover, the peptide helped increase skin thickness (density) for both morning and evening (Figure 3).
We have therefore been able to see in vivo, what was already observed both in vitro and ex vivo. The cyclic peptide, c[E5Ch-H], because it can increase calreticulin and BiP it is able to successfully increase TGFB3, collagen and protect from protein misfolding and therefore, presents a positive effect on wrinkles and skin thickness on volunteers.
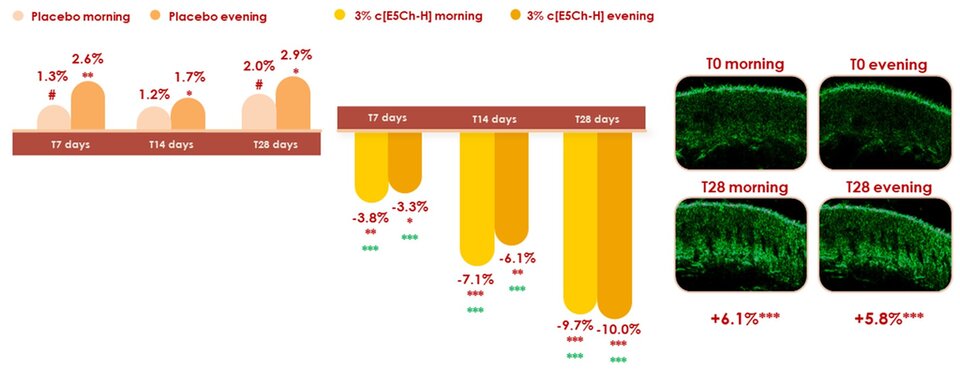
Figure 3. (Left) Bar graphs for wrinkle’s depth on crow’s feet (Primos 3D) after 7, 14 and 28 days of application (significance in green corresponds to vs placebo). (Right) Representative ultrasound images after 28 days of application of a cream containing 3% active ingredient and the corresponding overall improvement in skin thickness.
CONCLUSION
We have successfully synthesized a highly active cyclic peptide, c[E5Ch-H]. This peptide was originated from a proprietary linear hexapeptide and by performing cyclization, a novel active ingredient was obtained. The cyclic peptide was able to increase calreticulin and BiP, two chaperones involved in the UPR. Additionally, cell death was decreased after chemical induction of ER stress with Brefeldin A. Both activities were also confirmed in an ex vivo assay. Moreover, peptide c[E5Ch-H] was able to act on HEK-HDF communication by increasing TGFB3 and collagen synthesis in fibroblasts when treated with keratinocyte conditioned media. In a clinical assay, the active ingredient has shown to significantly decrease crow’s feet wrinkles and increase skin thickness in volunteers with aging signs. Taken all together, we can see a novel active ingredient with a clear anti-aging activity, a cyclic peptide, a not so common structure in the cosmetic industry.

Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.

Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Zompra AA, Galanis AS, Werbitzky O, Albericio F. Manufacturing peptides as active pharmaceutical ingredients. Future Med. Chem. 2009; 1, 361-377.
- Claro B, Bastos M, Garcia-Fandino R. Design and applications of cyclic peptides. Peptide Application in Biomedicine, Biotechnology and Bioengineering. 2018; 87-129.
- Walter, P., and Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011; 334, 1081–1086.
- Chadwick, SR., and Lajoie, P. Endoplasmic Reticulum Stress Coping Mechanisms and Lifespan Regulation in Health and Diseases. Front Cell Dev Biol. 2019; 21;7:84.
- Naidoo, N., Ferber, M., Master, M., Zhu, Y., and Pack, A. I. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J. Neurosci. 2008; 28, 6539–6548.
- Fields GB and Noble RL. Solid-phase peptide synthesis utilizing 9-fluorenylmethozycarbonyl amino acids. International Journal of Peptide and Protein Research. 1990; 35: 161-214.
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13:89-102.
- Pandya UM, Manzanares MA, Tellechea A, Egbuta C, Daubriac J, et al. Calreticulin exploits TGF-β for extracellular matrix induction engineering a tissue regenerative process. FASEB J. 2020; 34: 15849-15874.
- Van Duyn Graham L, Sweetwyne MT, Pallero MA, Murphy-Ullrich JE. Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking and processing into the extracellular matrix. J Biol Chem. 2010; 5: 7067-78.
- Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010; 24: 665-83.
- Li, JH, Huang, XR, Zhu, HJ, Johnson, R, Lan, HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003; 63(6): 2010–2019.
- Murata, H, Zhou, L, Ochoa, S, Hasan, A, Badiavas, E, Falanga, V. TGF-beta3 simulates and regulates collagen synthesis through TGF-beta1-dependen and independent mechanisms. J Invest Dermatol. 1997; 108(3):258-62.
- Tsukahara K, Moriwaki S, Hotta M, Fujimura T, Kitahara T. A study of diurnal variation in wrinkles on the human face. Arch Dermatol Res. 2004; 296:169-74.
- Tsukahara K, Takema Y, Moriwaki S, Fujimura T, Imokawa G. Dermal fluid translocation is an important determinant of the diurnal variation in human skin thickness. Br J Dermatol. 2001; 145:590-6.