
AI
Skin care
peer-reviewed
Precision Fermentation Meets AI: Transforming Personal Care Ingredient Sourcing and Innovation
NICK OUZOUNOV
Chief Technology Officer, Geltor, Inc., San Leandro, CA, USA
ABSTRACT: Ethical, environmental, or sourcing constraints have historically limited the availability of personal care ingredients. Precision fermentation, originally pioneered in medicine, has provided a new avenue for sourcing personal care ingredients that bypasses the limitations of traditional methods. Now, with breakthroughs in artificial intelligence, exemplified by AlphaFold, RoseTTAFold, and models like BindCraft, as well as advances in strain engineering, powered by models such as Evo 2, have unlocked a new era of innovation that is transforming the industry. These new technologies can significantly expand the accessible ingredient space for personal care, driving continued improvements in purity, scalability, and product performance. This article examines how advancements in precision fermentation, AI-driven protein design, and strain engineering are reshaping innovation in personal care by enabling sustainable production of high-performance ingredients that meet evolving consumer expectations.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
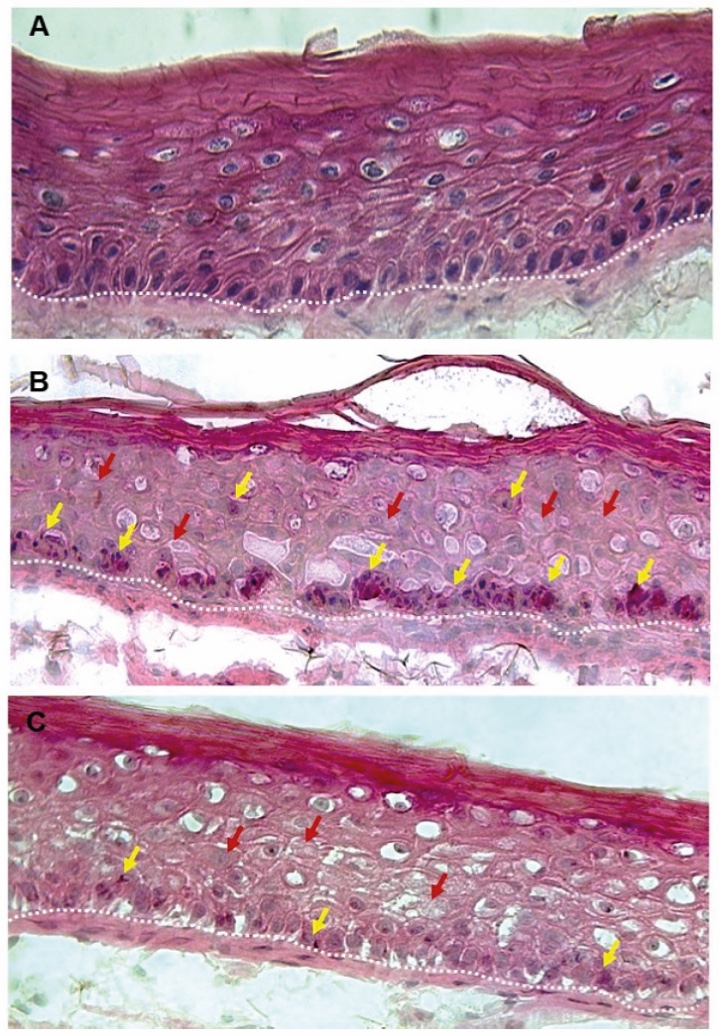
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Precision Fermentation for Sustainable Ingredient Solutions
While fermentation has been used for the production of products like beer, yogurt, and bread for millennia, most consider the start of precision fermentation (PF) to be in the late 1970s with its transformation of medicine. Genentech’s recombinant human insulin was one of the first major PF products. In 1978, Genentech genetically engineered bacteria to produce human insulin, replacing the old method of extracting insulin from pig and cow pancreases. This biosynthetic insulin (marketed as “Humulin”) proved to be as effective as insulin from the human pancreas while eliminating the allergic reactions caused by animal-derived insulin (1). The new process also solved supply issues—previously it took >50,000 animal pancreases to make one kilogram of insulin (2)—by enabling production at scale via fermentation tanks.
Beyond pharmaceuticals, precision fermentation has rapidly expanded into other markets like the beauty and personal care sector (3). Biotechnology now offers a way to “brew” high-value ingredients in microbes providing an alternative to sourcing them from animals or petrochemicals. Fermentation derived cosmetic actives are growing in demand as brands seek natural, sustainable, and high-performance ingredients. Perhaps the most notable example of this change is Hyaluronic acid (HA), valued for its hydration and viscoelastic properties in skincare. HA was originally obtained from animal tissues, notably rooster combs (4). For decades, companies extracted HA by processing tons of rooster combs, which contain the highest levels of this molecule found in animals. However, this method had downsides: it was costly, yielded limited quantities, and raised ethical and safety concerns (animal parts can introduce impurities or pathogens). HA from animal sources can also vary in quality and carries a risk of immune reactions if not purified thoroughly. Since the early 1990s, most HA has been produced via precision fermentation using microbes, eliminating the need for animal harvest (4, 5). Typically, strains of Streptococcus bacteria are engineered to secrete hyaluronic acid during fermentation. This shift to microbial production was driven by the need for a more controllable, scalable process—it’s easier to brew HA in tanks than to depend on slaughterhouse byproducts.
As with HA, precision fermentation has recently been used to produce proteins of interest such as collagen for personal care applications. Animal collagen production is resource-intensive, and can be linked to environmental degradation and deforestation, particularly in regions such as the Amazon, where livestock farming drives significant ecological damage. Investigations have revealed direct links between collagen sourcing and deforestation practices associated with major industry players, spotlighting an urgent need for sustainable alternatives (6, 7).
On top of the sustainability challenges, commercial collagen for food and cosmetics typically consists of only two fibrillar collagens types: type I and a small fraction of type III (8). For example, type I collagen constitutes around 85-90% of the organic content of skin, followed by type III collagen at less than 10%. These two types (I and III) are valued for their skin-plumping and structural benefits, but they represent just the “structural scaffold” collagens. This is because traditional methods of boiling skins and acid/enzymatic extraction cannot easily isolate other collagen types as those exist in much lower quantities or in hard-to-extract tissues (9, 10). In practice, this means nearly all collagen ingredients available until recently have been the same few types (mostly I, occasionally II or III), but most of the 28 collagens (which account for less than 3% of the total collagen pool) are not accessible in useful quantities from conventional animal sources.
Type 21 collagen is one of the more recently discovered collagen types. It was identified through the Human Genome Project in 2001 when researchers mining genomic data found a gene (COL21A1) encoding a collagen protein (11). Expression studies showed COL21A1 (type 21) is present in many tissues–including heart, skeletal muscle, stomach, skin, kidney, and placenta—and it is described as helping to maintain the integrity of the extracellular matrix (12, 13). A proteomic study of human dermis across ages found COL21A1 is highly expressed in toddler and young adult skin but drastically drops off with age. This reduction could contribute to the reduced dermal elasticity and impaired wound healing seen during aging (14). While Type 21 collagen is present at extremely low concentrations and nearly impossible to isolate from tissues, precision fermentation makes it possible to produce it in the quantities and purity needed for beauty and personal care applications regardless of its natural scarcity.
In conclusion, precision fermentation has fundamentally transformed ingredient sourcing for personal care by providing alternatives to traditional sourcing that can have limitations such as ethical concerns, environmental impacts, and ingredient scarcity.
From Rational Design to Nobel Prize-Winning AI
Early protein engineering was very laborious. It was heavily dependent on sourcing naturally occurring sequences, followed by extensive chemical mutagenesis and the screening of thousands of variants to identify beneficial changes. The first protein created entirely from scratch (de novo-designed protein) without relying on natural sequences was a four-helix bundle protein created by rational design in 1987. It was a landmark development that highlighted the initial potential of computational methods even though it was limited by computational constraints at the time (15, 16). In the 1990s, the landscape changed significantly with directed evolution, pioneered by Frances Arnold, who introduced iterative mutation and selection methods to evolve enzymes with enhanced or novel functionalities. Arnold's pioneering work was awarded the Nobel Prize in Chemistry in 2018, recognizing directed evolution as a powerful tool for enzyme and protein development (17).
Traditional computational approaches to protein engineering remained limited by predictive inaccuracies. Initial computational attempts at rational protein design typically had low success rates (often below 1%), requiring significant experimental validation (18). Over the subsequent decades, substantial improvements in computational accuracy were achieved, particularly with the development of sophisticated software like Rosetta, which facilitated early successes in protein structure modeling and design (19). Despite these advances, it was the introduction of artificial intelligence, especially deep-learning techniques, that dramatically changed the trajectory of protein engineering, significantly increasing the speed and accuracy of computational protein design.
Recent breakthroughs in AI-driven protein design, notably recognized by the 2024 Nobel Prize in Chemistry, have profoundly reshaped the biotechnology landscape. AlphaFold, developed by DeepMind, has provided scientists with a transformative tool capable of accurately predicting three-dimensional protein structures from amino acid sequences. Nobel Prize laureates Demis Hassabis and John Jumper revolutionized structural biology by developing AlphaFold, which provides researchers with computationally accurate structural predictions rapidly, and at an unprecedented scale (20).
Complementary advances such as RoseTTAFold, developed by David Baker's laboratory at the University of Washington, similarly harness deep-learning approaches to facilitate accurate predictions and de novo protein design (21). Baker’s work, also recognized with a Nobel Prize, allowed the design of novel proteins with specific functions and folds never previously observed in nature (20). These tools have dramatically improved success rates in protein engineering, from initial success rates of around 1% to contemporary design success rates approaching 10%–100%, depending on the specific protein library and design goals (22).
Moreover, innovative AI platforms such as BindCraft have further refined the capability of computational design by accurately predicting protein-ligand interactions and significantly enhancing specificity and binding affinity. BindCraft leverages the predictive power of AlphaFold2, enabling the rapid design of highly specific and potent protein binders without extensive high-throughput screening (22). Collectively, these developments have transitioned protein engineering from a predominantly empirical discipline to a highly precise computational science, unlocking previously unimaginable innovations in functional protein design.
The Evolution of Protein Engineering in Personal Care
Consumers and brands are increasingly seeking high-performance bioactive ingredients. Brands are eager to introduce novel protein peptides and polypeptides with unique benefits that can improve skin firmness, reduce wrinkles, or protect hair to differentiate their products. These bioactives are becoming a focal point for innovation, as they align with consumer preferences for clean, ethical beauty and promise enhanced efficacy compared to legacy ingredients.
Biotechnology companies are leveraging AI to design and discover these novel bioactive protein peptide and polypeptide ingredients at an unprecedented pace and many are using precision fermentation to sustainably produce them. Leaders in the space include companies like Geltor, Nuritas, and Shiru, that have developed internal AI models in their designs and actively work with other companies through partnerships (23, 24, 25).
The recent explosion of AI models for protein folding and design, both open-source and proprietary, is a key factor enabling new innovations. Importantly, many AI models and databases have been made publicly available, lowering barriers for innovation. DeepMind open-sourced the AlphaFold protein structure predictions for hundreds of millions of proteins, and academic groups have released open tools like ProteinMPNN (for sequence design given a structure) and diffusion models for protein generation. This means even smaller companies or research teams can leverage state-of-the-art AI in their ingredient discovery.
This growing availability of AI models is directly fueling innovation in the personal care market. Open tools let researchers identify new bioactive motifs or simulate protein-surface interactions quickly, inspiring novel ingredient concepts. Proprietary models, fine-tuned on specialized data (such as human skin proteomics, peptide datasets, or natural product libraries), enable companies to discover unique actives with specific benefits (like a polypeptide that triggers extracellular matrix production, or an enzyme that generates a new cosmetic compound). The result is a faster pipeline from idea to ingredient: AI can suggest completely new protein candidates in silico, which are then synthesized via fermentation and tested, shortening development cycles that once took years. We’re already seeing an uptick in AI-discovered personal care ingredients hitting the market and this is just the beginning (26).
Strain Engineering and Metabolic Pathway Optimization
Parallel to protein design advancements, artificial intelligence has also revolutionized strain engineering for microbial production hosts, significantly enhancing metabolic pathway optimization. A key factor in improving sustainability is improving strain performance in converting carbon sources like sugar into the target molecule being produced while limiting CO2 production. Traditional strain optimization relied on random mutagenesis and iterative trial-and-error, often consuming significant resources and time. Similarly to protein design, biotech companies have invested in developing internal proprietary models to aid in the strain improvement process but also utilize publicly available ones.
As an example, Evo 2 represents a major step forward in strain engineering due to its ease of use and extensive data foundation. Developed by the Arc Institute in collaboration with NVIDIA, Stanford University, UC Berkeley, and UC San Francisco, it was trained on an unprecedented dataset containing 9.3 trillion nucleotides from over 128,000 different organisms. Leveraging this massive dataset, Evo 2 can help more accurately analyze and design genetic modifications in order to optimize genomes, design novel genetic circuits, and redirect metabolic pathways (26).
This predictive capability has profound implications for sustainability and scalability. By quickly pinpointing precise genetic changes, models like Evo 2 can significantly reduce the traditional strain engineering timelines by decreasing the need for extensive laboratory trial-and-error approaches. These targeted genetic adjustments can enable production of ingredients with higher yields, improved consistency, and reduced environmental footprint.
Importantly, models like Evo 2 are often open-source which democratizes this powerful technology, enabling even small biotechnology companies and research teams to more rapidly enhance their production strains, accelerate innovation, and further improve their carbon footprint (27).
Challenges and Opportunities
Precision fermentation and AI offer enormous potential to more sustainably produce previously scarce, ethically complex, or completely novel ingredients. This innovation aligns with consumer demands for clean, high-performance ingredients, reshapes markets, and reduces environmental impacts. However, significant challenges remain. Regulatory frameworks must also balance rapid innovation with proactive oversight to manage biosecurity risks (28). Scaling PF processes from lab to commercial volumes frequently encounters hurdles like decreased yields, fermentation inefficiencies, and resource intensity.
Historical examples from pioneering companies like Zymergen illustrate how AI-driven success in the lab often faces significant barriers in commercial production (29). Ingredient manufactures looking to utilize precision fermentation should prioritize standardizing fermentation and downstream purification processes capable of producing diverse proteins using the same infrastructure and process. Companies like Geltor exemplify this approach, having developed standardized precision fermentation and downstream purification protocols that enable manufacturing diverse protein targets using the same scaled production process (23). Such uniformity significantly reduces development timelines from years to months, allowing facilities to rapidly switch between product targets and accelerate time to market.
As the integration and expectations around AI intensify, careful management of the substantial electrical and computational requirements is critical (30). Companies must ensure that the implementation of AI tools genuinely leads to tangible benefits, such as improved sustainability or efficiency, rather than inadvertently increasing carbon emissions or resource consumption. Ultimately, achieving the full promise of precision fermentation and AI-driven synthetic biology requires a balanced approach, combining innovation-friendly regulatory frameworks, sustainable scaling practices, and efficient technological integration.
Conclusion
In conclusion, precision fermentation, together with advancements in AI-driven protein design and strain engineering, is continuing to transform how we create and source ingredients for personal care. These technologies can provide scalable, sustainable solutions to traditional challenges, enabling the rapid development of novel, bioactive ingredients. While hurdles remain, the use and democratization of powerful AI models along with precision fermentation offer tremendous opportunities for innovation, efficiency, and sustainability, positioning the personal care industry for unprecedented growth and consumer satisfaction.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
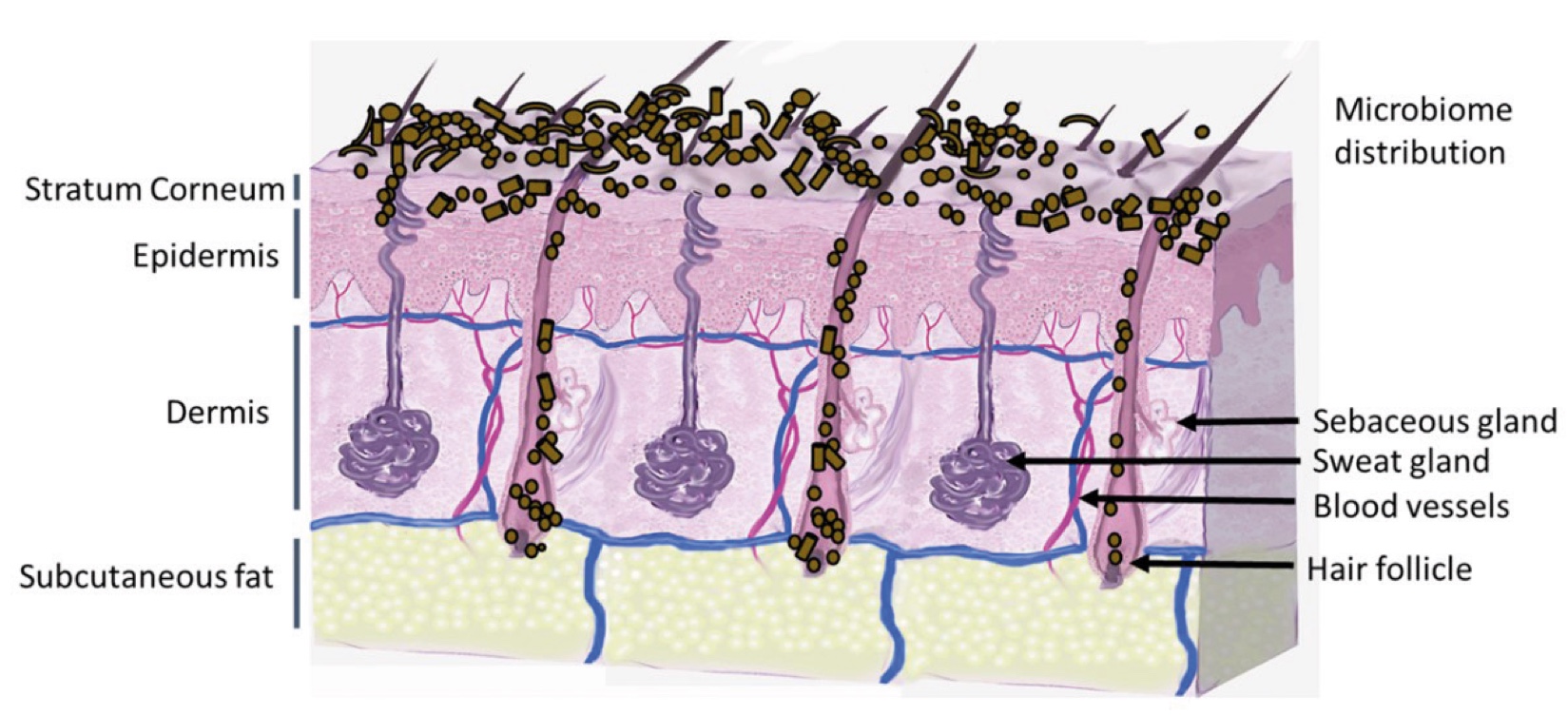
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- RethinkX. The disruption of insulin [Internet]. RethinkX; 2020 Jan 9 [cited 2025 Mar]. Available from: https://www.rethinkx.com/blog/the-disruption-of-insulin
- Fraser L. Cloning insulin [Internet]. South San Francisco (CA): Genentech; 2016 Sep 6 [cited 2025 Mar]. Available from: https://www.gene.com/stories/cloning-insulin
- Xylome. How biotech is transforming beauty: replacing palm oil with fermented alternatives – free webinar [Internet]. Cosmetics & Toiletries; 2024 Nov 22 [cited 2025 Mar]. Available from: https://www.cosmeticsandtoiletries.com/cosmetic-ingredients/natural-sustainable/news/22927253/xylome-how-biotech-is-transforming-beauty-replacing-palm-oil-with-fermented-alternatives-free-webinar
- Serra M, Casas A, Toubarro D, Barros AN, Teixeira JA. Microbial hyaluronic acid production: a review. Molecules. 2023 Feb 23;28(5):2084. doi: 10.3390/molecules28052084. PMID: 36903332; PMCID: PMC10004376.
- Walker K, Basehore BM, Goyal A, et al. Hyaluronic Acid [Internet]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023 Jul 3 [updated 2023 Jul 3; cited 2025 Mar]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482440/
- Pitman S. Greenpeace says beauty industry is contributing to Amazon destruction [Internet]. CosmeticsDesign-Europe; 2009 Jun 1 [updated 2017 Mar 18; cited 2025 Mar]. Available from: https://www.cosmeticsdesign-europe.com/Article/2009/06/02/Greenpeace-says-beauty-industry-is-contributing-to-Amazon-destruction
- Mendonça E, Wasley A, Zuker F. Collagen craze drives deforestation and rights abuses [Internet]. The Bureau of Investigative Journalism; 2023 Mar 6 [cited 2025 Mar]. Available from: https://www.thebureauinvestigates.com/stories/2023-03-06/collagen-wellness-industrys-star-product-drives-deforestation-and-rights-abuses
- Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011 Jan;3(1):a004978. doi: 10.1101/cshperspect.a004978. PMID: 21421911; PMCID: PMC3003457.
- Jadach B, Mielcarek Z, Osmałek T. Use of collagen in cosmetic products. Curr Issues Mol Biol. 2024 Mar 4;46(3):2043–70. doi: 10.3390/cimb46030132. PMID: 38534748; PMCID: PMC10968853.
- Bielajew BJ, Hu JC, Athanasiou KA. Collagen: quantification, biomechanics, and role of minor subtypes in cartilage. Nat Rev Mater. 2020 Oct;5(10):730–47. doi: 10.1038/s41578-020-0213-1. Epub 2020 Jul 20. PMID: 33996147; PMCID: PMC8114887.
- Fitzgerald J, Bateman JF. A new FACIT of the collagen family: COL21A1. FEBS Lett. 2001 Sep 14;505(2):275–80. doi: 10.1016/s0014-5793(01)02754-5. PMID: 11566190.
- Chou MY, Li HC. Genomic organization and characterization of the human type XXI collagen (COL21A1) gene. Genomics. 2002 Mar;79(3):395–401. doi: 10.1006/geno.2002.6712. PMID: 11863369.
- Dyring-Andersen B, Løvendorf MB, Coscia F, Santos A, Møller LBP, Colaço AR, et al. Spatially and cell-type resolved quantitative proteomic atlas of healthy human skin. Nat Commun. 2020 Nov 5;11(1):5587. doi: 10.1038/s41467-020-19383-8. PMID: 33154365; PMCID: PMC7645789.
- Li M, Li X, Liu B, Lv L, Wang W, Gao D, et al. Time-resolved extracellular matrix atlas of the developing human skin dermis. Front Cell Dev Biol. 2021 Nov 26;9:783456. doi: 10.3389/fcell.2021.783456. PMID: 34901026; PMCID: PMC8661536.
- Rennie J. Three biochemists win Chemistry Nobel for directing evolution [Internet]. Quanta Magazine; 2018 Oct 3 [cited 2025 Mar]. Available from: https://www.quantamagazine.org/frances-arnold-george-smith-and-gregory-winter-win-chemistry-nobel-for-directing-evolution-20181003/
- Regan L, Caballero D, Hinrichsen MR, Virrueta A, Williams DM, O'Hern CS. Protein design: Past, present, and future. Biopolymers. 2015 Jul;104(4):334–50. doi: 10.1002/bip.22639. PMID: 25784145; PMCID: PMC4856012.
- Nobel Prize Outreach. Frances H. Arnold – Facts – Nobel Prize in Chemistry 2018 [Internet]. NobelPrize.org; [cited 2025 Mar]. Available from: https://www.nobelprize.org/prizes/chemistry/2018/arnold/facts/
- Bennett NR, Coventry B, Goreshnik I, Huang B, Allen A, Vafeados D, et al. Improving de novo protein binder design with deep learning. Nat Commun. 2023 May 6;14(1):2625. doi: 10.1038/s41467-023-38328-5. PMID: 37149653; PMCID: PMC10163288.
- Kuhlman B. Designing protein structures and complexes with the molecular modeling program Rosetta. J Biol Chem. 2019 Dec 13;294(50):19436–43. doi: 10.1074/jbc.AW119.008144. Epub 2019 Nov 7. PMID: 31699898; PMCID: PMC6916497.
- Nobel Prize Outreach. Press release – Nobel Prize in Chemistry 2024 [Internet]. NobelPrize.org; 2024 Oct 2 [cited 2025 Mar]. Available from: https://www.nobelprize.org/prizes/chemistry/2024/press-release/
- Krishna R, Wang J, Ahern W, Sturmfels P, Venkatesh P, Kalvet I, et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science. 2024 Apr 19;384(6693):eadl2528. doi: 10.1126/science.adl2528. Epub 2024 Apr 19. PMID: 38452047.
- Pacesa M, Nickel L, Schmidt J, Pyatova E, Schellhaas C, Kissling L, et al. BindCraft: one-shot design of functional protein binders. bioRxiv [Preprint]. 2024 Oct 2 [cited 2025 Mar];2024.09.30.615802. doi: 10.1101/2024.09.30.615802.
- Geltor. Platform [Internet]. San Leandro (CA): Geltor; [cited 2025 Mar]. Available from: https://geltor.com/platform/
- DeSmit O. Newly A.I. discovered peptide promises anti-aging results [Internet]. CosmeticsDesign-USA; 2023 Sep 28 [cited 2025 Mar]. Available from: https://www.cosmeticsdesign.com/Article/2023/09/28/Newly-A.I.-discovered-peptide-promises-anti-aging-results
- Protein Discovery, Inc. [Internet]. Protein Discovery; [cited 2025 Mar]. Available from: https://proteindiscovery.ai/
- Weber M. Skin & hair care’s protein-based revolution [Internet]. Global Cosmetic Industry Magazine; 2024 Aug 26 [cited 2025 Mar]. Available from: https://www.gcimagazine.com/brands-products/skin-care/article/22918741/skin-hair-cares-proteinbased-revolution
- Helmy M, Smith D, Selvarajoo K. Systems biology approaches integrated with artificial intelligence for optimized metabolic engineering. Metab Eng Commun. 2020 Dec;11:e00149. doi: 10.1016/j.mec.2020.e00149. Epub 2020 Oct 9. Erratum in: Metab Eng Commun. 2021 Oct 28;13:e00186. doi: 10.1016/j.mec.2021.e00186. PMID: 33072513; PMCID: PMC7546651.
- Undheim TA. The whack-a-mole governance challenge for AI-enabled synthetic biology: literature review and emerging frameworks. Front Bioeng Biotechnol. 2024 Feb 28;12:1359768. doi: 10.3389/fbioe.2024.1359768. PMID: 38481570; PMCID: PMC10933118.
- Blois M. Zymergen reveals layoffs following setbacks for first product [Internet]. Chemical & Engineering News; 2021 Oct 28 [cited 2025 Mar]. Available from: https://cen.acs.org/business/biobased-chemicals/Zymergen-reveals-layoffs-following-setbacks/99/i40
- Cho R. AI’s growing carbon footprint [Internet]. State of the Planet, Columbia Climate School; 2023 Jun 9 [cited 2025 Mar]. Available from: https://news.climate.columbia.edu/2023/06/09/ais-growing-carbon-footprint/
