
Skin Care
Skin care
peer-reviewed
Soothing and Protective effects of a mixture of Saccharide Isomeratem and D-panthenol on skin vascular homeostasis
JIYING DONG1, SHANSHAN YAO1, WENJUAN ZHANG2, JIAO SONG2, TING DOU2, NAN LU2*
* Corresponding author
- Department of Plastic and Reconstructive Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiaotong University of Medicine, Shanghai, China
- Shanghai Kanghua Times Biomedical Technology Co., Ltd,Shanghai, China
ABSTRACT: Disruptions in vascular homeostasis can precipitate deleterious consequences, including vascular barrier disruption, endothelial dysfunction, altered vascular permeability, aberrant blood flow dynamics, and vasodilation. Inflammatory aging, skin sensitivity, redness, and other skin problems are the destructive consequences of vascular homeostasis imbalance. Redness of the facial skin is an important cosmetic concern. In this study, we aim to evaluate the beneficial effects of a novel cell protective agent, 9thOSMO (a mixture containing 1% Saccharide Isomeratem and 2% D-panthenol) in alleviating LPS-induced vascular homeostasis imbalance. And evaluate its soothing and protective effects on red skin from multiple dimensions through human clinical trials.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction
Vascular homeostasis is an important basis for body life activities, including normal structure and function of vascular cells (1), platelet adhesion (2) and inhibition of blood coagulation, accurate regulation of vascular tension (3). Vasoactive substances are an important part of vascular homeostasis regulation, which precisely regulate the structure and function of blood vessels (4). The vascular endothelium, acting as a selective biological barrier, is essential for vascular and skin health, influencing processes such as permeability, angiogenesis, and immunity (5). Its well-defined barrier structure prevents extravasation of fluids, ions, and immune cells, while signaling pathways adapt dynamically to meet physiological demands.
Recent evidence highlights the importance of endothelial cell contacts (6), particularly adherens junctions (AJ) (7, 8, 9), in maintaining vascular barrier function. VE-cadherin, a key component of AJ, is critical for endothelial cell connections and barrier architecture (5, 10), regulating vascular permeability and influencing signaling pathways that affect endothelial cell behavior (11, 12).
The endothelium also plays a vital role in vascular tone regulation through the release of vasodilatory factors such as nitric oxide (NO), primarily produced by eNOS (13). This helps maintain vascular wall stability by preventing thrombosis, cell proliferation, and inflammation. However, factors like inflammation and oxidative stress can disrupt vascular homeostasis (14), leading to endothelial dysfunction, increased permeability, and downregulation of inflammatory cytokines such as COX-2 (15) and IL-8 (16). These changes contribute to skin issues like redness, aging, and sensitivity.
The redness of the cheeks plays an important role in perceptions of health and attractiveness for both genders, and having a healthy appearance is universally desired. When redness is caused by oxygenated blood, the perceivers judge redder faces as healthier; thus, facial redness may reflect the cardiovascular health of individuals (17). However, redness of the facial skin beyond a certain degree can be perceived as unhealthy and induces self-embarrassment. Under normal skin physiology, the skin maintains a healthy appearance as the body's microcirculation delivers oxygen and nutrients to skin cells while removing metabolic waste to ensure proper functioning. When the skin is exposed to external stimuli, changes in microcirculation can trigger inflammatory responses. The release of inflammatory mediators causes blood vessels to dilate and increases fluid leakage from blood vessels, leading to redness in the skin. Therefore, facial skin redness is an important cosmetic concern (17).
9thOSMO is a cell protective agent based on the technological achievements of Shanghai Ninth People's Hospital, which is a clear, yellowish to slightly amber colored and viscous liquid but is water-soluble with the main active ingredients 1% corn-derived Saccharide Isomeratem and 2% D-panthenol. Saccharide Isomeratem is 100% natural and plant-based D-glucose, which has potential anti-inflammatory applications (18), and a certain moisturizing (19) effect in cosmetics (20). D-panthenol,a precursor of vitamin B5, has demonstrated anti-inflammatory activity by suppressing the production of pro-inflammatory molecules (21, 22).
On this basis, compared with the current research on keratinocyte or fibroblast models, this article takes the source (blood vessels) that causes skin redness as the research object, and evaluates the stabilizing effect of 9thOSMO on vascular homeostasis by regulating the permeability, barrier function, and inflammatory factors of endothelial cells. Additionally, in clinical trials, the improvement effect of 9thOSMO on skin redness is evaluated from three different perspectives: anti-irritation, soothing, and irritability protection.
Materials and Methods
In vitro studies
Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were purchased from Shanghai Zhongqiaoxinzhou Biotech (China). HUVECs were cultured in endothelial cell basal medium (EGM-2; Lonza, USA) supplemented with EGM-2MV at 37℃ and 5 % CO2. Cells between passages 3 and 8 were used for the experiments.
Cytotoxicity Measurement
The cytotoxicity of 9thOSMO, which is soluble in the EGM-2,was determined using 3-[4,5-dimethylthiazol-2-y-2,5-diphenyltetrazolium bromide (MTT) reduction to the corresponding. Briefly, cells (6*103 cells/well) were inoculated in 96-well plates for 24h before being exposed to 9thOSMO (0%, 0.4 %,0.6 %,0.8 %,1 %,1.4 %,1.8 %,2 %) in humidified 5 % CO2 at 37 ℃ for 12 h, followed by the addition of reagent containing MTT reagent (10 μL) each well. After 4 h, DMSO (100μL) was added to dissolve the formed formazan salt, and then the absorbance of each well was measured at 570 nm using a microplate reader. Viability was expressed as a percentage of the control, which without any treatment (0% 9thOSMO).
Endothelial Permeability
HUVECs (5*104 cells/well) were seeded on gelatin-coated insert membranes (Sigma, USA) with 0.4 μm-diameter pores and grown in 12 multi-well plates for 72 h. Confluent monolayers were pre-treated with LPS (2μg/ml, 12 h), then add fresh culture medium with 0.5 % 9thOSMO or vehicle (only medium) for 12 h. Throughout the treatments, the cells were maintained in a medium with 10 % FBS. FITC-Dextran (3-5kDa, 10 μg/mL) was used as a fluorescent probe of permeability. At various time intervals (e.g., 0, 5, 15, 30, 45, 60, 120, and 180 min after treatment), fluorescence in the lower compartment was measured (485 and 535 nm excitation and emission, respectively) by using a microplate reader. Data are reported as fluorescence intensity.
Immunofluorescence staining
HUVECs (5*104 cells/well) were seeded on gelatin-coated 96-well plate for 72 h. Confluent monolayers were pre-treated with LPS (2μg/ml, 12 h), then added fresh culture medium with 0.5 % 9thOSMO or vehicle (only medium) for 12 h. Cells were washed with PBS and fixed with 4 % paraformaldehyde for 15 min at room temperature, washed with PBS for 10 min, and blocked with 5 % bovine serum albumin in PBST (0.3 % Triton X-100 in PBS) for 1 hour at room temperature. The cells were incubated overnight at 4°C with the following primary antibodies: anti–mouse VE-Cadherin antibody (1:100, Sino Biological). The cells were washed sufficiently with PBS to remove unbound antibody and incubated with secondary antibodies (cy3-conjugated anti-rabbit IgG) for 60 min at room temperature. Nuclei were stained with DAPI. Visualized
by fluorescence microscopy.
Quantitative Reverse Transcription PCR
HUVECs (156K/cm2) were seeded on gelatin-coated 12-well plate for 72 h. Confluent monolayers were treated with LPS (2μg/ml, 12 h), then added fresh culture medium with 0.5 % 9thOSMO or vehicle (only medium) for 12 h. Total RNA was extracted from these cells using TRIzol RNA isolation kit (Vazyme, China) according to the manufacturer’s guidelines and was reverse-transcribed into cDNA using a PrimeScriptTM RT reagent cDNA Synthesis Kit (Takara, Japan). Quantitative PCR was performed using TB SYBR Green Premix Ex TaqTMⅡ (Takara, Japan). β-actin was used as an internal reference in each reaction. Each sample was run in triplicate, and the relative gene expression was analyzed using the 2ΔΔCt. The sequences of the primers are listed in Table 1.
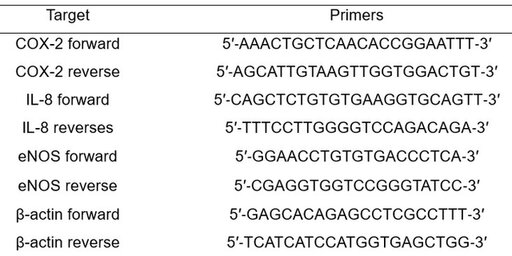
Table 1. Primers of COX-2, IL‐8, eNOS , and β-actin for qRT‐PCR.
Clinical trials
Human Skin Anti-Irritation Test
Thirty-two healthy Chinese subjects were selected on the basis of inclusion and exclusion criteria, and written consent was obtained in each case. The average age was 46.4 years (range 29–58: 2 males and 30 females). The subjects had no history of allergic contact dermatitis, nor had they used topical or systemic irritant preparations in the previous month. Sodium lauryl sulfate (SLS, 0.1%) and SLS containing 9thOSMO (1%, 3%, and 5%) formulated with aqueous solution were prepared and applied. The patches (chambers) stayed in back for 24 h. Once the patches were removed, a reading was done 0.5, 24 and the 48 h later; readings were scored according to the 2015 version of China's "Safety and Technical Standards for cosmetic” guidelines as follows: 0 = no reaction; 1 = Suspected response, only slight erythema; 2 = Weak positive reaction (erythema reaction); erythema, infiltration, edema, with possible papules; 3 = Strong positive reaction (herpes reaction); erythema, infiltration, edema, papules, herpes; reaction may extend beyond the test area; 4 = Extremely strong positive reaction (coalescent vesicle reaction); marked erythema, severe induration, edema, coalescent vesicles; reaction beyond the test area.
Skin Soothing and Protective Effect of 9thOSMO
Thirty subjects (average age: 35.47±3.86, 1 male and 29 females) participated in this study. The erythema was induced on the forearms with 0.3% Methyl nicotinate (MN) (Adamas-beta, China). The 2 test sites were MN irradiated. One applied 2% 9thOSMO; another as a negative control. The erythema index was evaluated using a Mexameter® MX18 (Courage + Khazaka electronic GmbH, Cologne, Germany) and the image was captured by VISIA-CR (Canfield, USA).
Erythema index and VISIA-CR capture were performed before MN stimulation (baseline), immediately after MN stimulation (T0), 30 minutes (T30), and 60 minutes. Note: T0 is 15-20 minutes after MN stimulation; Apply the 9thOSMO after T0 testing, T30 and T60 are all time points after sample application. In addition, after the complete disappearance of the erythema (about 3-4 hours after MN stimulation), immerse the calibration areas of the left and right arms completely in a 42 ℃ constant temperature water bath for 3 minutes. The erythema index and VISIA-CR images were collected before and after the water bath. Note, the data collection time after the hot water bath is about 5 minutes after the arm leaves the bath, and the other non-treated areas of the forearm turn red and fade away.
Statistical Analysis
All data were expressed as mean ± SD. Differences between the control and treatment group were evaluated by Student’s t-test using the GraphPad Prism 6.0 or IBM SPSS Statistics 22.0. A p < 0.05 was considered statistically significant.
Results
Cytotoxicity
HUVECs were treated with 9thOSMO at different concentrations (0 %, 0.4 %, 0.6 %, 0.8 %,1.0 %, 1.4 %, 1.8 %, and 2 %) for 24 h. As shown in Figure 1, 9thOSMO (0–0.6 %) had no cytotoxic effect on HUVECs after 24 h incubation (Figure 1).
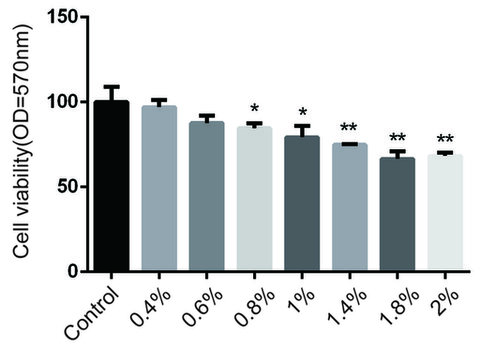
Figure 1. Effect of 9thOSMO on HUVEC viability. Data are the mean±SEM of three independent experiments. “*” indicates a significant difference from the Control (* p<0.05, **p<0.01).
Effect of 9thOSMO on Endothelial Permeability in Response to LPS
Inflammatory injury of the endothelium leads to endothelial dysfunction. In order to investigate the contribution of 9thOSMO for endothelium integrity, a permeability assay was performed on confluent HUVEC monolayers exposed to LPS. LPS significantly increased endothelial permeability compared with the control condition. The incubation of endothelial monolayers with 0.5 % 9thOSMO reduced LPS-induced hyperpermeability, showing a repair effect (Figure 2A).
To further strengthen our findings, the immunofluorescence analysis of tight junction proteins in endothelial cells was also performed. VE-Cadherin was evaluated as a representative marker of endothelial tight junctions. In the basal control condition, HUVECs confluent monolayer expressed VE-Cadherin with a plasmalemmal localization at cell–cell contacts, as well as cytoplasmatic and perinuclear accumulation. After treatment with LPS, gaps appear between adjacent endothelial cells, correlating increased permeability with endothelial gap formation. The incubation of endothelial monolayers with 0.5 % 9thOSMO decreased LPS-induced gaps (Figure 2B).
Collectively, these data indicate that 9thOSMO maintains the endothelial integrity and repairs the acute injury induced by LPS on the barrier function of the endothelium.
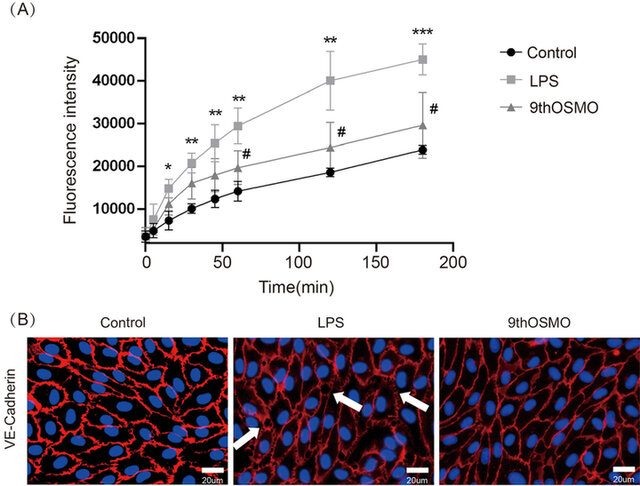
Figure 2. Effect of 9thOSMO on endothelial hyperpermeability induced by LPS. (A) FITC-dextran transport in the lower compartment was measured at the end of LPS stimulation; (B) Integrity of cell–cell contacts evaluated by immunofluorescence analysis of VE-Cadherin in HUVEC monolayers. Data are the mean±SEM of three independent experiments. “*” indicates a significant difference from the Control (*p<0.05, **p<0.01 , ***p<0.001). “#” indicates a significant difference from the 9thOSMO (#p<0.05).
Effect of 9thOSMO on the gene expression of eNOS, COX-2, IL-8
The expressions of two recognized proinflammatory mediators, COX-2 and IL-8, were estimated to investigate the activity of 9thOSMO in the process of inflammation-related gene expressions. The data showed that after LPS induction followed by treatment with 0.5% 9thOSMO, the expression of LPS-induced COX-2 and IL-8 was downregulated, especially IL-8 expression, which was comparable to the control group (Figure 3A,3B). In order to gain insights into endothelial-mediated effects, eNOS mRNA was assessed. The mRNA expression of eNOS was significantly reduced by LPS. When treated with 0.5 % 9thOSMO after LPS induction, eNOS mRNA expression was significantly enhanced, and comparable to the control group expression, with no statistical difference (Figure 3C).
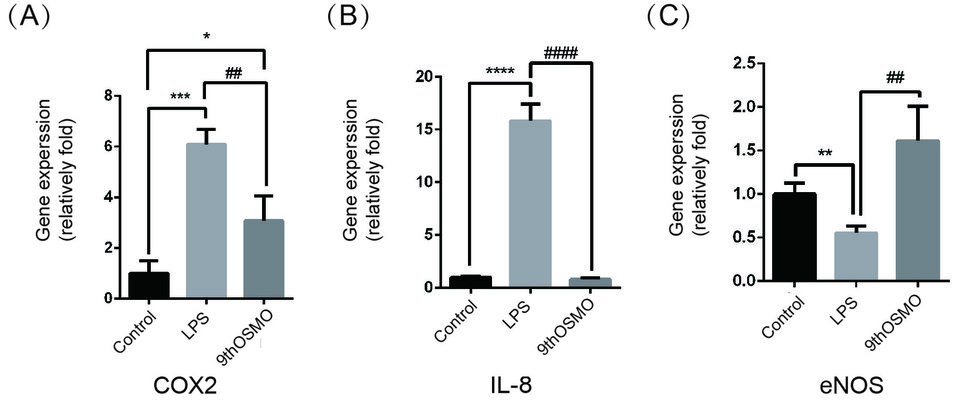
Figure 3. Effect of 9thOSMO on COX-2 (A), IL-8 (B), and eNOS (C) expressions in LPS-treated cells.Data are the mean±SEM of three independent experiments. “*” indicates a significant difference from the Control (*p<0.05 , **p<0.01,***p<0.001, ****p<0.0001). “#” indicates a significant difference from the 9thOSMO (##p<0.01, ####p<0.0001).
Human Skin Anti-Irritation Test of 9thOSMO
A patch test was performed to evaluate the anti-irritation effect of 9thOSMO for clinical applications to human skin. As shown in Table 2 and Figure 4. 1% 9thOSMO-SLS, the number of adverse reactions was significantly less than 0.1% SLS at 48h, the score of adverse reactions was significantly lower than that of 0.1% SLS at 24h and 48h (Figure 4a and 4d); 3% 9thOSMO-SLS, the number and the score of adverse reactions were both significantly less/lower than 0.1% SLS at 48h(Figure 4b and 4e); 5% 9thOSMO-SLS, number and score of adverse reactions were both significantly less/lower than 0.1% SLS solution at 0.5, 24 and 48h (Figure 4c and 4f).
The data showed that 1% 9thOSMO could alleviate the adverse reactions caused by SLS, with the main onset time being 24 hours after removing the stimulus and lasting until 48 hours; the soothing effect of 3% 9thOSMO takes effect within 48 hours; and 5% 9th OSMO takes effect within 0.5 hours and can last for at least up to 48 hours.
These results indicate that 9th OSMO can reduce/mitigate the probability and degree of adverse skin reactions caused by SLS stimulation.
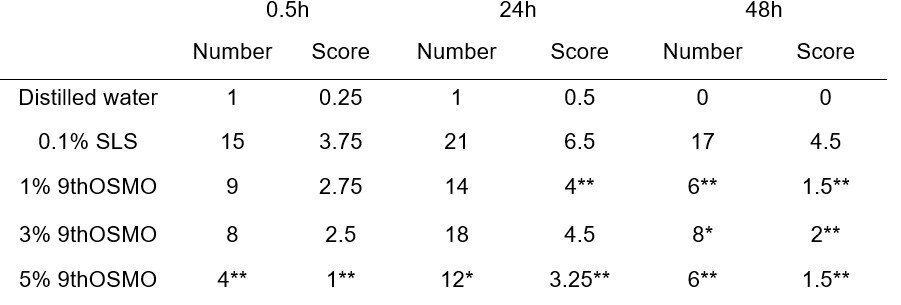
Table 2. Human skin primary anti-irritation test.
Score = Sum of the scores of 32 subjects. *p<0.05 , **p<0.01, 9thOSMO vs 0.1% SLS. Number: non-parametric Wilcoxon matched pairs signed-ranks test; Score: Pearson's Chi-squared test
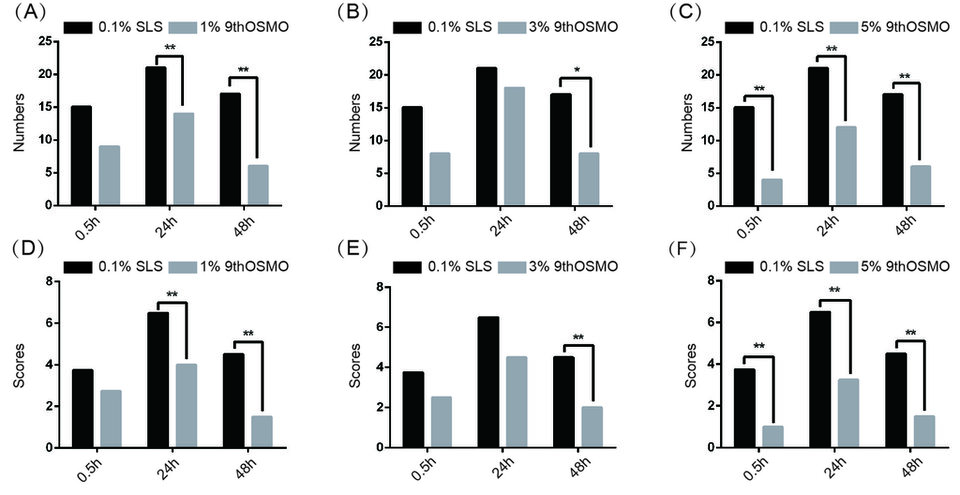
Figure 4. Number and score of skin adverse reactions by 9thOSMO-SLS. (A)-(C) represents the number of adverse reactions after applying different concentrations of 9thOSMO. (D)-(F) represents the scores of adverse reactions after applying different concentrations of 9thOSMO.*p<0.05, **p<0.01 , ***p<0.001).
Skin Soothing and Protective Effect of 9thOSMO
Based on the above experiments and considering the effectiveness and cost, this study chose 2% as the applied dose. The skin Soothing effect of 9thOSMO was evaluated in vivo using the MN-induced. The erythema index (EI) significantly increased when exposed to MN. Then with the increase of time, EI showed a trend of first increasing and then decreasing. Relative to the baseline, 60 minutes after applying 2% 9thOSMO, the EI was significantly lower than the negative control (without applying any products) (Table 3 and Figure 5). This indicates that the 2% 9thOSMO has a significant effect in reducing skin redness, and can speed up the rate at which the skin's redness subsides (16.52% vs negative control; 22.20% vs T0).
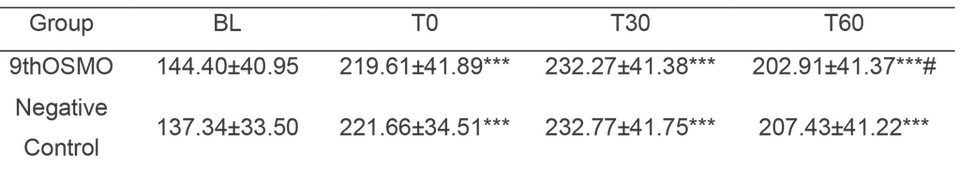
Table 3. The value of skin EI at different time points. Data are presented as the mean ± SD.
***p < 0.001 compared with the BL, # p < 0.05 compared with the Negative group.
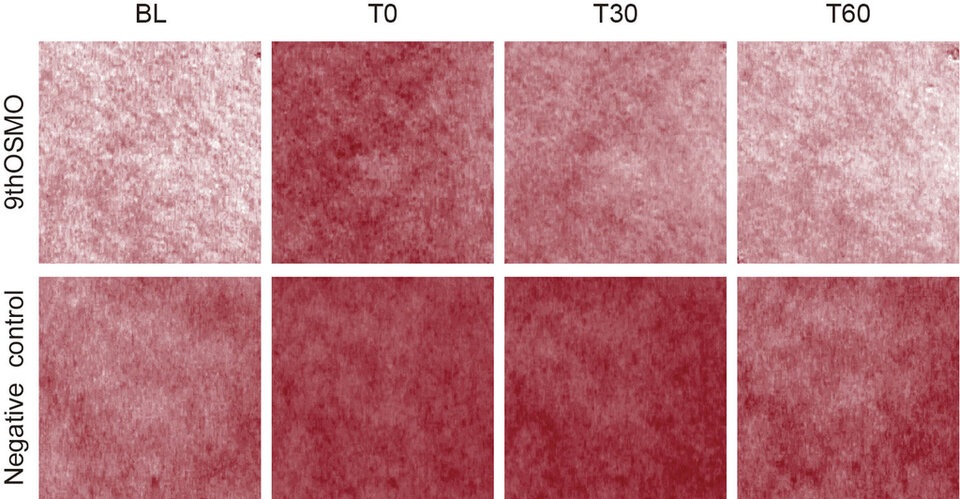
Figure 5. VISIA-CR images of erythema on forearm at different time points.
The skin Protective effect of 9thOSMO on Irritable Skin was also evaluated in vivo using the MN and hot water stimulation test. This experiment first observed skin redness with MN, then applied the 2% 9thOSMO; after the skin erythema faded, use 42℃ water to stimulate the original MN area, observes the erythema of the 9thOSMO and the negative control group, and evaluates the protective effect of the 9thOSMO on easily irritated skin when facing external stimuli again.
Compared to before hot water stimulation, after MN stimulation, when the skin in the area where 2% 9thOSMO was applied faced with hot water stimulation again, the EI was significantly lower than the negative control group (Table 4 and Figure 6). This indicates that a 2% 9thOSMO has a protective effect on the redness phenomenon of easily irritated skin when facing re-stimulation (Protection rate 25.38%).

Table 4. The value of skin EI at different time points. Data are presented as the mean ± SD
***p < 0.001 compared with the BL;# p < 0.05, indicates that the difference between before and after-heating compared to the negative control.
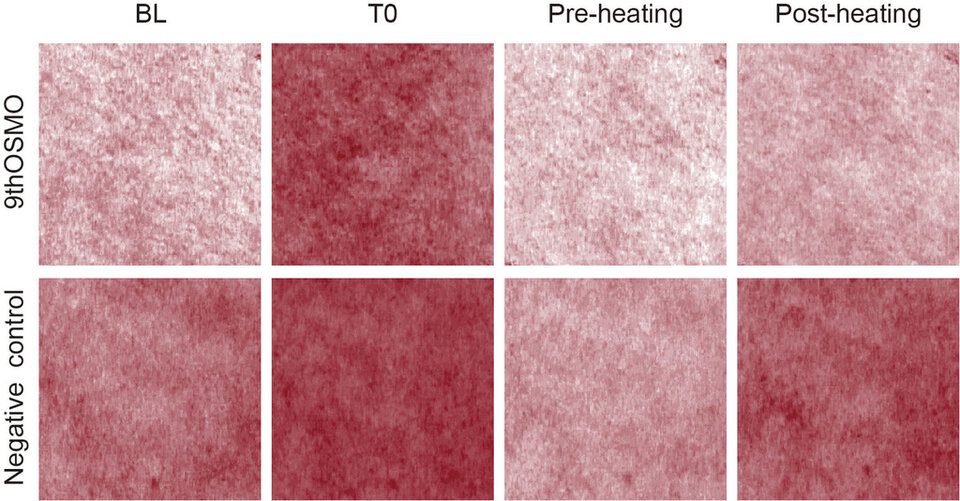
Figure 6. VISIA-CR images of erythema on forearm at different time points.
Discussion
In this study, the effects of 9thOSMO on Vascular permeability and skin redness associated effects were determined using various models. In vitro experiments use lipopolysaccharide (LPS) as an inflammatory response inducer. The LPS-induced in vitro inflammation model shares similarities with human skin inflammatory diseases (e.g., atopic dermatitis, psoriasis) in terms of inflammatory factor release and skin barrier function disruption. VE-Cadherin immunofluorescence staining and FITC-dextran permeability experiments demonstrated that the exposure of HUVECS to the pro-inflammatory stimulus LPS led to a significant increase in endothelial permeability. The incubation of endothelial monolayers with 9thOSMO decreased LPS-induced gaps. It suggests that this 9thOSMO might contribute to the maintenance of vascular integrity and functionality under inflammatory conditions.
Then, the LPS induced an increase of typical markers of inflammation, COX-2 and IL-8. 9thOSMO significantly down-regulates COX-2 and IL-8 mRNA expression in HUVECs, indicating its potential use as an anti-inflammatory agent in the prevention of inflammation. Meanwhile, 9thOSMO can significantly up-regulate the expression of eNOS that is down-regulated by LPS in HUVECs, proving its repairing endothelial cell function. And D-panthenol may alleviate inflammation by reducing COX-2, and Saccharide Isomerate may relieve inflammation by modulating the cytoskeleton arrangement via glycolysis regulation.
In human clinical trials, the Anti-Irritation and soothing effects of active substances are mainly evaluated through two models: SLS and MN stimulation. SLS, a surfactant frequently used in the induction of experimental irritant contact dermatitis in animals and in humans, which could penetrates into the skin, causes skin irritation associated with alterations of epidermal differentiation comprising the down-regulation of pro-filaggrin and the desquamation-related enzymes, kallikreins 5 and 7, and excess production of two inflammatory mediators, IL-1 and PGE2 (23). MN is a nicotinic acid, which induces a local cutaneous erythema when topically applied to the skin. Its rubefacient effect is related to a transient increase in the microcirculatory perfusion, which is hypothesized to be mediated through the prostaglandin D2 (PGD2) pathway. The mechanisms of action are, however not presently completely elucidated, and like with most compounds that cause vasodilation, the effect is likely to be of multifactorial origin, including release of nitric oxide (NO) from the endothelium, and neural effects (24). In the irritation experiment induced by SLS, the results indicate that compared to 0.1% SLS, 9thOSMO (1%, 3%, and 5%) - SLS solutions can significantly reduce the number and score of adverse reactions. 1% 9thOSMO takes effect within 24 hours, 3% 9thOSMO takes effect within 48 hours, and 5% 9thOSMO takes effect within 0.5 hours, with the effect lasting up to 48 hours. Additionally, The MN-induced skin redness test showed that the application of 2% 9thOSMO can accelerate the regression of skin redness. Compared to the Negative Control,theredness was 16.52% lower after 60 min.
This article further investigates the protective effect of the 9thOSMO on Irritable Skin. First, skin redness is induced by MN, then the sample is applied. After the erythema faded, hot water was used to simulate external stimulation the previously reddened area, and the degree of skin redness was observed. The results showed that 2% 9thOSMO has a good protective effect on the redness phenomenon of easily irritated (fragile) skin when facing re-stimulation. Compared to not applying anything, the sample has a skin protection rate of 25.38%.
The results indicate that the 9thOSMO can reduce vascular permeability, improve endothelial cell function, decrease the expression of pro-inflammatory factors, and maintain vascular homeostasis. And it has significant anti irritation and soothing effects, , better protecting easily irritated skin, improving skin redness.
Conclusion
The data acquired in this study demonstrated that 9thOSMO can maintain vascular homeostasis by reducing vascular permeability, repairing endothelial function, and reducing the expression of pro-inflammatory factors. Therefore, 9thOSMO can serve as a potential active ingredient to improve skin manifestations caused by vascular homeostasis imbalance (due to inflammation, Medical Aesthetics, etc.), such as sensitivity (redness, swelling, itching, pain), aging, etc. In clinical trials, it can significantly counteract external stimuli and soothe the skin. The most surprising aspect is that, in addition to alleviating the irritation caused by stimuli on normal skin, the 9th OSMO can also protect fragile skin in a state of irritation.
Therefore, 9thOSMO acts as an effective therapeutic agent, offering soothing and protective benefits for sensitive or fragile skin after medical aesthetic procedures.
Acknowledgment
CONFLICT OF INTEREST STATEMENT
There are no potential conflicts of interest to disclose.
DATA AVA ILAB IL ITY STATEMENT
All data generated or analyzed during this study are included in this. Further enquiries can be directed to the corresponding author.
ETHICS STATEMENT
The subjects involved in the human trial in this article have all signed informed consent forms
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications
The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Kruger-Genge, A.; Blocki, A.; Franke, R. P.; Jung, F., Vascular Endothelial Cell Biology: An Update. Int J Mol Sci 2019,20 (18).http://dx.doi.org/10.3390/ijms20184411
- Xu, X. R.; Carrim, N.; Neves, M. A.; McKeown, T.; Stratton, T. W.; Coelho, R. M.; Lei, X.; Chen, P.; Xu, J.; Dai, X.; Li, B. X.; Ni, H., Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb J 2016,14 (Suppl 1), 29.http://dx.doi.org/10.1186/s12959-016-0100-6
- Grandoch, M.; Bollyky, P. L.; Fischer, J. W., Hyaluronan: A Master Switch Between Vascular Homeostasis and Inflammation. Circ Res 2018,122 (10), 1341-1343.http://dx.doi.org/10.1161/CIRCRESAHA.118.312522
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y., The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther 2023,8 (1), 217.http://dx.doi.org/10.1038/s41392-023-01481-w
- Immanuel, J.; Yun, S., Vascular Inflammatory Diseases and Endothelial Phenotypes. Cells 2023,12 (12).http://dx.doi.org/10.3390/cells12121640
- Vestweber, D.; Broermann, A.; Schulte, D., Control of endothelial barrier function by regulating vascular endothelial-cadherin. Curr Opin Hematol2010,17 (3), 230-6.http://dx.doi.org/10.1097/MOH.0b013e328338664b
- Friedl, P.; Mayor, R., Tuning collective cell migration by cell–cell junction regulation. Cold Spring Harbor perspectives in biology 2017,9 (4), a029199
- Ono, S.; Egawa, G.; Kabashima, K., Regulation of blood vascular permeability in the skin. Inflammation and regeneration2017,37, 1-8
- Pugsley, M. K.; Tabrizchi, R., The vascular system: An overview of structure and function. Journal of pharmacological and toxicological methods2000,44 (2), 333-340
- Gavard, J., Breaking the VE-cadherin bonds. FEBS Lett 2009,583 (1), 1-6.http://dx.doi.org/10.1016/j.febslet.2008.11.032
- Gavard, J., Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adh Migr 2014,8 (2), 158-64.http://dx.doi.org/10.4161/cam.29026
- Pober, J. S.; Sessa, W. C., Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 2007,7 (10), 803-15.http://dx.doi.org/10.1038/nri2171
- Bachschmid, M. M.; Schildknecht, S.; Matsui, R.; Zee, R.; Haeussler, D.; Cohen, R. A.; Pimental, D.; Loo, B., Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med 2013,45 (1), 17-36.http://dx.doi.org/10.3109/07853890.2011.645498
- Chang, L.; Garcia-Barrio, M. T.; Chen, Y. E., Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol2020,40 (5), 1094-1109.http://dx.doi.org/10.1161/ATVBAHA.120.312464
- Zhang, Z.; Li, L.; Huang, G.; Zhou, T.; Zhang, X.; Leng, X.; Chen, Z.; Lin, J., Embelia Laeta aqueous extract suppresses acute inflammation via decreasing COX-2/iNOS expression and inhibiting NF-κB pathway. Journal of Ethnopharmacology 2021,281, 114575
- Matsushima, K.; Yang, D.; Oppenheim, J. J., Interleukin-8: An evolving chemokine. Cytokine 2022,153, 155828
- Jones, A. L.; Porcheron, A.; Sweda, J. R.; Morizot, F.; Russell, R., Coloration in different areas of facial skin is a cue to health: The role of cheek redness and periorbital luminance in health perception. Body image 2016,17, 57-66.http://dx.doi.org/10.1016/j.bodyim.2016.02.001
- Hou, C.; Chen, L.; Yang, L.; Ji, X., An insight into anti-inflammatory effects of natural polysaccharides. International journal of biological macromolecules 2020,153, 248-255
- Polaskova, J.; Pavlackova, J.; Vltavska, P.; Mokrejs, P.; Janis, R., Moisturizing effect of topical cosmetic products applied to dry skin. J Cosmet Sci 2013,64 (5), 329-340
- Vlorensia, H. H.; Abdullah, H.; Martinus, A. R.; Ikhtiari, R., The Effect of a Moisturizing Cream with Saccharide Isomerate and Ceramide on Increasing Skin Hydration. 2020,
- Li-Mei, W.; Jie, T.; Shan-He, W.; Dong-Mei, M.; Peng-Jiu, Y., Anti-inflammatory and Anti-oxidative Effects of Dexpanthenol on Lipopolysaccharide Induced Acute Lung Injury in Mice. Inflammation2016,39 (5), 1757-63.http://dx.doi.org/10.1007/s10753-016-0410-7
- Tan, C.; Peng, K.; Lim, T.; Liu, J.; Ye, Y.; Lim, L.; Gao, P.; Oblong, J. E.; Lam, T., The combination of allantoin, bisabolol, D-panthenol and dipotassium glycyrrhizinate mitigates UVB-induced PGE(2) synthesis by keratinocytes. Int J Cosmet Sci2024.http://dx.doi.org/10.1111/ics.12951
- Mizutani, T.; Mori, R.; Hirayama, M.; Sagawa, Y.; Shimizu, K.; Okano, Y.; Masaki, H., Sodium Lauryl Sulfate Stimulates the Generation of Reactive Oxygen Species through Interactions with Cell Membranes. Journal of oleo science 2016,65 (12), 993-1001.http://dx.doi.org/10.5650/jos.ess16074
- Elawa, S.; Mirdell, R.; Tesselaar, E.; Farnebo, S., The microvascular response in the skin to topical application of methyl nicotinate: Effect of concentration and variation between skin sites. Microvasc Res 2019,124, 54-60.http://dx.doi.org/10.1016/j.mvr.2019.03.002
