
Regulation
Skin care
peer-reviewed
Trends and Developments in Substantiating Clinical Claims
RANIA IBRAHIM, Ph.D.
Founder SkinScience Analytics, USA
ABSTRACT:The substantiation of clinical claims in the cosmetics and personal care industry has traditionally relied on standardized methodologies, such as structured surveys and conventional instrumentation. However, these approaches often fall short in capturing the nuanced, emotional, and physiological responses of consumers. The article advocates for a paradigm shift towards more innovative and holistic testing methods. One emerging trend is the integration of neurofeedback testing, which measures physiological indicators like brain activity and heart rate to assess genuine consumer reactions to products. This method provides deeper insights into user experiences that traditional surveys may overlook. Additionally, the application of Artificial Intelligence (AI) in data analysis offers the potential to process vast datasets, enabling more accurate predictions of product safety and efficacy. These advancements align with regulatory developments which emphasizes the need for scientifically robust methods in safety substantiation. By embracing neurofeedback and AI-driven analytics, the industry can enhance the reliability of clinical claims, meet evolving regulatory standards, and better cater to consumer expectations.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
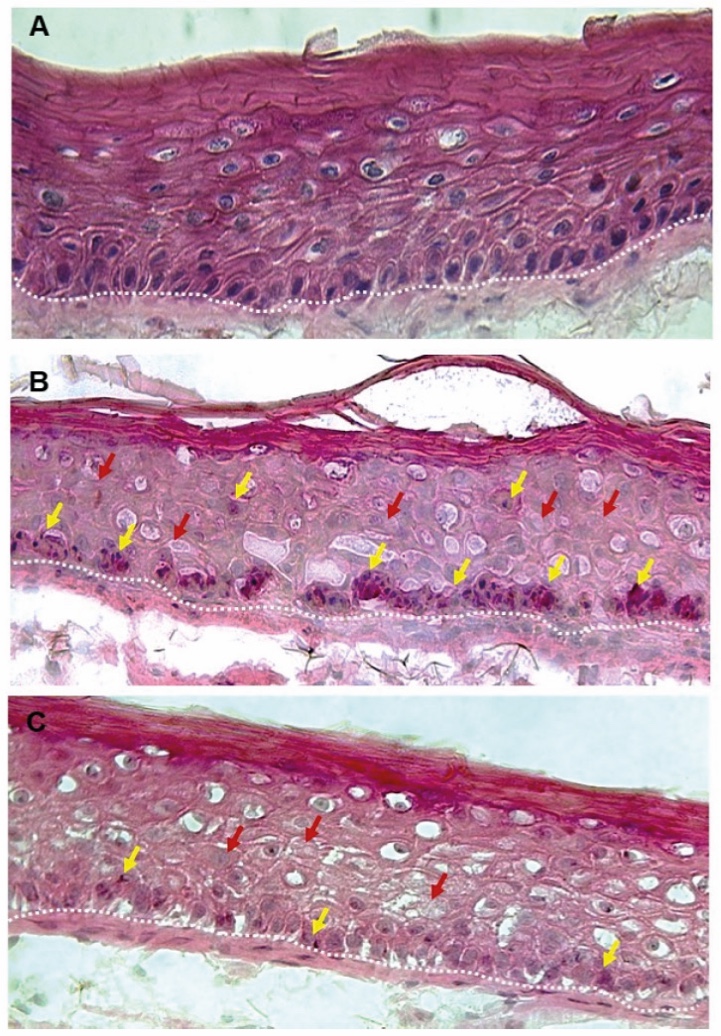
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Innovation Deficit in Clinical Claims Testing Methodologies
The evolution of clinical testing methods to substantiate claims has been progressing at a protracted pace. Testing labs need to more effectively leverage technologies and measurement tools that will meet manufacturer as well as consumer needs. Currently, most clinical testing labs use the same standard instruments and the same protocols with minimal innovation. A more thorough implementation of innovative instruments and testing protocols is necessary. Methodologies used to substantiate and assess claims remain standardized through instruments and surveys to grade attributes on the face or skin. The cosmetics testing industry needs to be seeking more profound input from the participant’s experience. This deeper perspective provides a more holistic approach to testing, in an era where consumers are more emotionally attached to products. Measurement of those emotions and the consumer experience with the product is evolving to an unprecedented level of insight.
Limitations of Survey Research
Traditionally widely practiced methods of gauging consumer response are through surveys to describe their experience. Does the skin feel more radiant? Do trial participants agree, disagree, or strongly disagree? They are being asked to respond in highly structured scales, to articulate their feelings about a product with the limitation of responding to language on a survey. On the other hand, neurofeedback provides data that measures the actual feeling, the emotion, how the brain and heart rate are changing, as well as sensory factors. It examines how the body is physically reacting to a product. Not all clinical trial participants are capable of fully expressing their experience with the product in writing. Most participants don't want to write an essay after they just answered a long list of questions with a yes or no response. While open-ended questions exist on surveys and provide important data, there can be more accurate gauges to the psychology as well. A major limitation of surveys is participants may feel reluctant to provide a negative response due to a perceived risk they may not be invited to participate in other clinical trials. Responses to consumer surveys should not necessarily be the only measure of safety and efficacy. Even if thousands of participants unequivocally say that the product experience was amazing, when you supplement that with data of how they react to the product from a physiological standpoint, insight from those aggregated results will inevitably be more powerful.
Current Trend: Neurofeedback testing
Neurofeedback testing is emerging as a novel method for evaluating product efficacy by measuring physiological responses like brain activity and heart rate, offering more authentic and insightful data than traditional consumer surveys. The ability to gain access to innovative assessment methods of consumer responses to products will be a significant industry trend moving forward. Neuroscience driven testing of skin products and makeup evaluates consumer experiences and behavior towards brands and products, which provides not only more inclusive data on product safety and efficacy but valuable strategic insights into marketing decisions. (1). Neurocosmetics explores innovations that can potentially enhance physical appearance and contribute to psychological well-being. It represents a holistic approach that integrates neuroscience into skincare Measurement tools in cosmetics testing include electrocardiogram (ECG), electroencephalography (EEG), facial electromyography, mouse monitoring, eye tracking, galvanic skin response (GSR), implicit tests and Virtual Reality.
The testing industry’s current standard protocols do not include the measurement of consumer physiological responses to products, for example the emotions invoked when our skin feels hydrated when we use a product. It is possible to measure how firm the skin is and the depth of the wrinkle and its structure. However, evaluating emotional responses in a physiologic way is highly valuable data. When an emotional response is measured, it will provide user feedback on a whole new level. Neurofeedback would capture consumer euphoria with the product that participants would not necessarily be able to articulate sufficiently in words on an open-ended survey question or a yes/no response to question prompts. What neurofeedback allows is a different way to substantiate your claims. (2)
Neurofeedback testing introduces innovative and expansive ways for completing the holistic representation of assessing how consumers are experiencing the product (3) which is a crucial mechanism of an effective consumer trial and provides a more complete picture beyond simple efficacy. Clinical claims including anti-aging products, long wear and water-resistant makeup, attributes associated with photo damage, fine lines and wrinkles, dullness of the skin, hyperpigmentation, firming, plumping of the skin or elasticity could be more rigorously tested and substantiated with the inclusion of neurofeedback.
Regulatory Compliance in Safety & Efficacy Testing
In the United States, there are shifts that impact the extent to which the beauty industry is held to regulatory standards. Innovation in testing will further enhance regulatory compliance by providing extensive data to support claims. Companies are held responsible by governments to ensure that if their product is on a physical or virtual shelf, then it must be safe and their company has appropriate checks and balances in place. Not only is this an ethical responsibility, but it is essential to marketing and distribution strategy. Platforms such as Amazon scrutinize their products before authorization to list products for sale. Whether companies are large, established manufacturers or a small, independent skincare line, if the products are being sold on Amazon or other global large scale distribution platforms, there is a requirement of safety reports for legal and liability reasons.
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) (4) enacted under the Biden Administration, requires all cosmetic companies in the United States to ensure and maintain rigorous and current records supporting adequate safety substantiation of their cosmetic products. Manufacturers are mandated to provide relevant safety data to support the safety of their products. According to MoCRA, it’s important that all data used to support the safety are derived from scientifically robust methods. Neurofeedback testing is the next frontier in redefining federally regulated scientifically robust methods.
Current Trend: AI Data Analysis in Safety & Efficacy Claims
Artificial Intelligence (AI) has the potential to revolutionize testing, as the technology can access and effectively analyze tremendous amounts of data sets for safety or efficacy. It is important to capitalize on access to data that can accurately predict which ingredients tend to cause more irritation or more reactions. With the data sets, clinical labs can have an algorithm that mines this data for them. AI data mining and analysis in terms of safety is a valuable predictor for companies. Currently, when clinical trials or safety tests are commissioned, they are performing a safety test in a small subset of people which represents a fraction of what might happen in the wider reality of a global consumer base.
From an efficacy perspective, analyzing data sets of specific, active ingredients observed in clinical trials over time, AI is being used for identifying these claims. For example, claims where consumers can look 10 years younger after using the product. After using the product for 8 weeks, the claim is you will look younger. That's how AI and its algorithms and data sets can substantiate to a reasonable extent. The software can generate a number based on your current photo and say you are 74. Then you use the product for 4 weeks, take another image, and then the software assesses the photo aging. Perhaps they look the same or look younger, depending upon whether the product improved the skin. With the vast amounts of data in terms of age-related photos that AI algorithms have access to, the technology can provide an elevated level of substantiating claims.
AI would supplement safety through the ability to mine the datasets of potential consumers to provide a predictor for the manufacturer. Testing is regularly conducted on a relatively small subset of participants, but the millions and millions of data sets that have been collected over the years and aggregated by AI could provide is that the chances of your product showing a reaction are very slim. AI supplements what most clinical labs can achieve in terms of numbers in human testing.
There are many trends to look forward to in clinical testing. Neurofeedback and AI are two prominent ones to track and keep current in our industry. It is our collective responsibility to uphold these standards and ensure that manufacturers and consumers enjoy the best possible experience with products, regardless of a small independent scale or large corporate scale.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
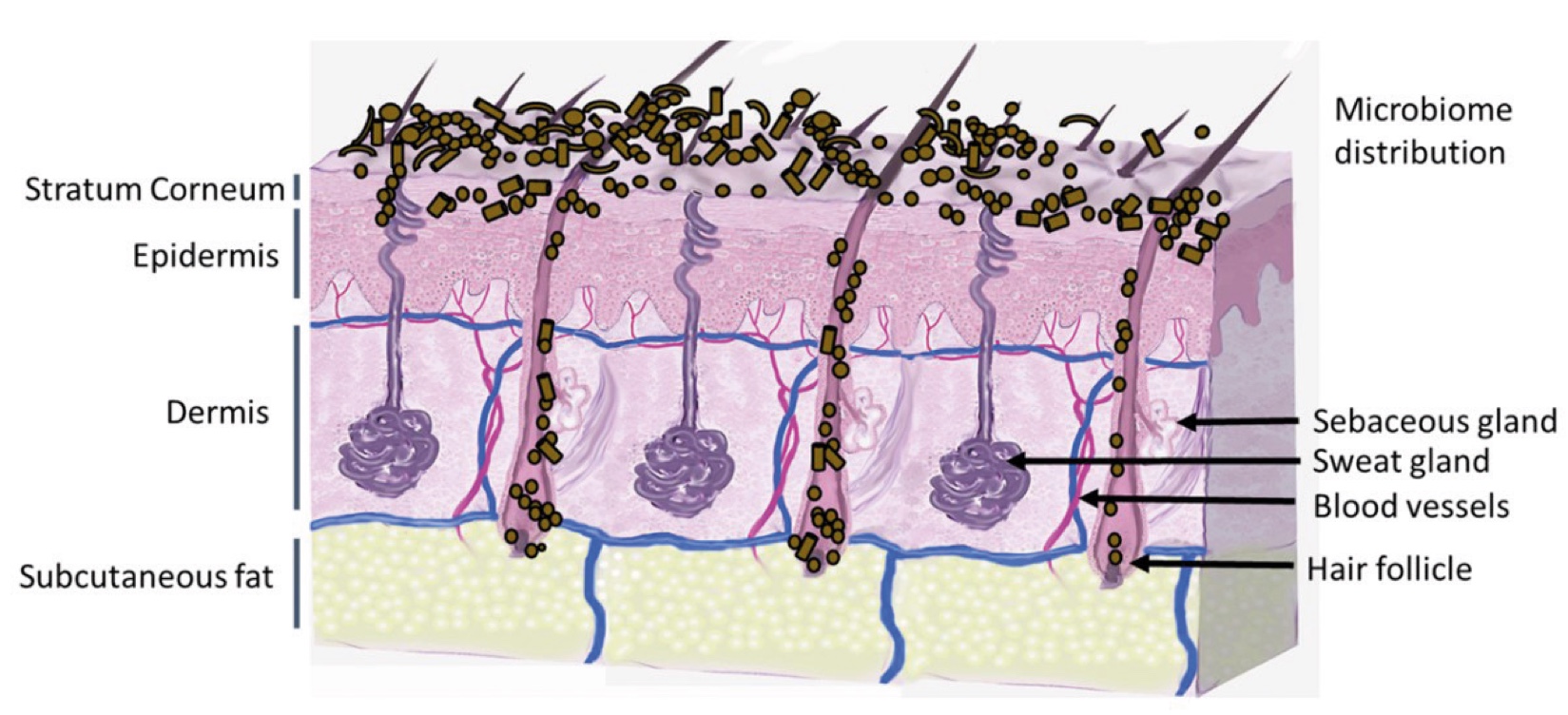
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Singh A, Chauhan SB, Singh I. Neurocosmetics: Exploring the intersection of neuroscience and cosmetics. Current Cosmetic Science. 2025 Feb 14;04. doi:10.2174/0126667797354459250204052908
- Rizzi V, Gubitosa J, Fini P, Cosma P. Neurocosmetics in skincare—the fascinating world of Skin–Brain Connection: A review to explore ingredients, commercial products for skin aging, and cosmetic regulation. Cosmetics. 2021 Jul 16;8(3):66. doi:10.3390/cosmetics8030066
- Ghalamghash S, Ghalamghash R. From brain to skin: Neurocosmetics pave the way into a no-cosmetics future. Regenerative Engineering and Translational Medicine. 2025 Feb 12; doi:10.1007/s40883-025-00390-4
- Commissioner O of the. Modernization of Cosmetics Regulation Act of 2022 [Internet]. FDA; [cited 2025 May 22]. Available from: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra
