Exploring Organic Acid Preservatives in Cosmetics
MALTE SIETZEN
Head of R&D and Quality Management, Evident ingredients GmbH, Germany
ABSTRACT: Cosmetics have come a long way from ancient beauty rituals to the high-tech formulations of today. But one thing remains constant—products need preservatives to stay safe and effective. With the rising demand for natural and sustainable ingredients, organic acids are stepping into the spotlight. This paper explores their chemistry, antimicrobial properties, benefits and sourcing options.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”

Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction
As consumers lean towards natural and clean beauty products, cosmetic chemists are on a mission to find s safer, milder and more sustainable preservatives. Enter organic acids naturally occurring compounds with a history of use in food and pharmaceuticals. From benzoic acid in cranberries (1) to sorbic acid in rowan berries (2), these compounds have naturally developed and been keeping microbes at bay for centuries. But how do they fare in modern cosmetic formulations? To answer this question, we first need to understand the chemistry of organic acids, and how it relates to their antimicrobial efficacy.
Why Do Organic Acids exhibit a pH-Dependent Antimicrobial Efficacy?
Organic acids are molecules containing carboxyl functional groups, which means they are available in their two complementary forms: the free acids (HA) and their corresponding salts (A-). When in aqueous solution, these two forms are in an equilibrium, meaning there are always both forms present, with the ratio being determined by the solution pH. It is essential to understand the equilibrium between these two forms , as this is the key factor in determining their antimicrobial potency. To this end it is important to realize that only the free acid form (HA) can effectively enter microbial cells due to its neutral charge, allowing it to diffuse across the lipid bilayer of the cell membrane (3). Once inside, the acidic environment of the cytoplasm causes the acid to partially dissociate, releasing protons and lowering the cell’s internal pH. This acidification disrupts enzyme activity, metabolic processes, and protein function, ultimately leading to microbial cell death (4). On the other hand, the ionized form (A-) carries a negative charge and is unable to pass through the hydrophobic cell membrane effectively. This is exemplified in Fig. 1 for sorbic acid/potassium sorbate.
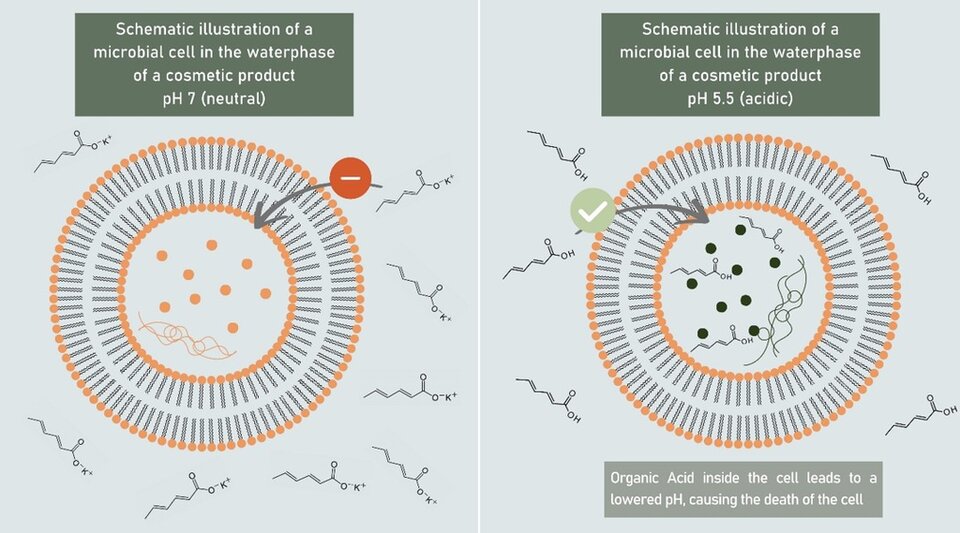
Figure 1. Charged Potassium Sorbate ions (left) are unable to penetrate the microbial membrane, while neutral sorbic acid (right) is able to pass that barrier.
After establishing that only the free acid HA is useful to us – we should find a way to estimate, and ideally calculate, the amount of HA that is available in solution under specific conditions. For this purpose, the Henderson-Hasselbalch equation (5) is a fundamental tool, as it describes acid-base equilibria by the following equation:

Rearrangement of the formula gives us the ratio of the concentrations of undissociated free acid [HA] and dissociated salt [A-] as a function of solution pH and the acid dissociation constant, pKa.
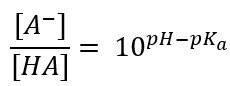
pKa is a substance-specific, solvent-dependent constant and, at least for a solution in pure water, the pKa values for common organic acids are well known. With this data in mind, we can easily plot the percentage of available free acid (HA) as a function of pH for various organic acids. For this plot we chose commonly known examples used for the preservation of cosmetic formulations, namely Citric Acid (pKa = 3.1), Lactic Acid (pKa = 3.9), Benzoic Acid (pKa = 4.2), p-Anisic Acid (pKa = 4.5), Levulinic Acid (pKa = 4.7), Sorbic Acid (pKa = 4.8) and Dehydroacetic Acid (pKa = 5.3) (6).
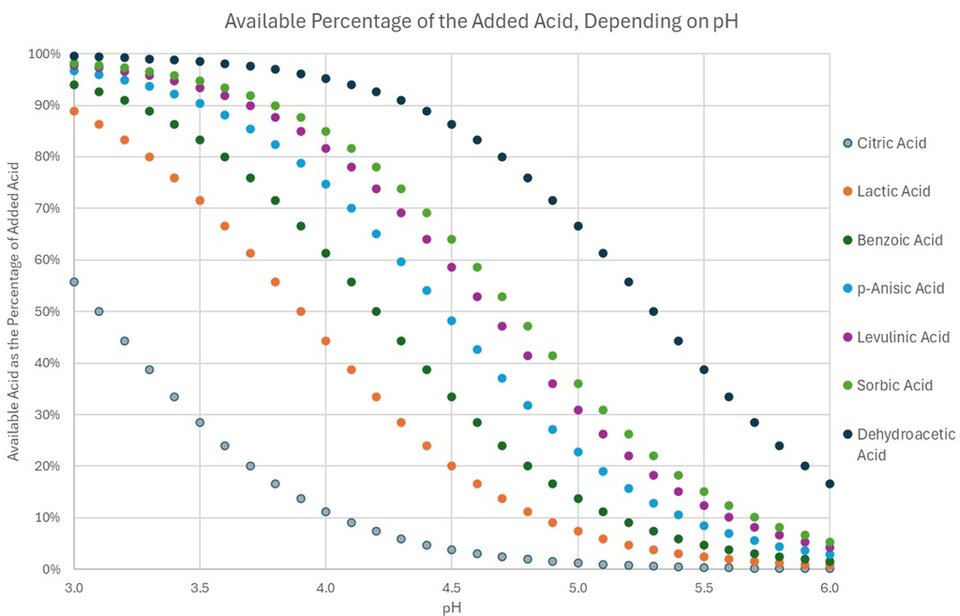
Figure 2. Graphical representation of the acid-base-equilibrium of various acids as a function of pH.
This plot is highly useful and should be studied by anyone who is considering working with organic acids, as it visualized the pH limitation that is inherent to this class of compounds. For example, in an aqueous solution of Benzoic Acid at pH 5.5, only about 4.8% of the used ingredient will be present as the free acid HA, and thus be available for its intended use of preserving the formulation, while the remaining 95.2% are inactive. However, this rather high percentage should not be considered as “dead weight”, as its presence constitutes a constant pressure to form more Benzoic Acid, after the originally active 4.8% have been either used up through decomposition or removal from the equilibrium, for example by diffusion into the bacterial cell.
With all this being said it is important to reconsider the solvent dependency of the dissociation constant pKa, as mentioned above. The pKa values used for this graph are strictly only valid for pure water, and thus are only qualitatively, but not quantitatively, applicable to cosmetic formulations. So while the principle established so far is true and is useful to explain organic acid behavior in cosmetic formulations, the exact numerical values calculated above should only serve as a trend, and will in reality be different. Any addition to the aqueous solution will change the pKa constant, and thus, concurrently, the equilibrium between HA and A-. But in which manner will it change? We can speculate that all of the common cosmetic ingredients are less polar than water and will hence lower the polarity of the aqueous phase. As a common chemical principle, this will stabilize the less polar side of the equilibrium, i.e. the undissociated form HA, and in this manner will lead to it’s improved availability (7).
To visualize this, it can be imagined as shifting the curves in Fig. 2 to the right, meaning that a higher percentage of the free acid HA will be available at any pH when compared to the solution in pure water.
It should be noted that the decrease in formulation polarity, while being a natural by-product of any formulation development, can also be used deliberately. The purposeful addition of glycols, surface active compounds, or similar will thus enhance the availability of the undissociated form HA and in turn increase the antimicrobial potency, as explained above.
Solubility and Application
After establishing the pH-dependency of the antimicrobial efficacy of organic acids, it is important to consider the second important factor, the solubility. This is a key factor in formulation technique —after all, what good is a preservative if it doesn’t dissolve properly? As it turns out, for organic acids the solubility is also pH dependent. Since some organic acids, such as Benzoic Acid or sorbic acid, exhibit limited solubility in aqueous solutions, the temporary transformation into their sodium or potassium salts to enhance solubility in aqueous formulations is necessary.
To improve solubility of organic acids that can’t be brought into solution, it is necessary to increase the pH to about 8, temporarily transforming the acids into the more soluble conjugate bases. After the required solubility is achieved the formulation’s pH can be lowered to 5.0-5.5 again, regenerating the acids and bringing them into their antimicrobial active state.
Sourcing – Petrochemical vs. Natural
Traditionally, most commercially available organic acids, have been synthesized from petrochemical sources, ensuring high purity and cost-effectiveness. However, growing consumer demand for natural and sustainable cosmetic ingredients has driven advancements in biotechnology and green chemistry, enabling the production of organic acids from renewable sources. Modern techniques, such as microbial fermentation and bio-based synthesis, allow for the extraction of these acids from plant-derived feedstocks, fruits, or bacterial cultures, reducing reliance on petrochemical substances. This shift not only aligns with the clean beauty movement but also enhances the marketability of cosmetics labeled as "natural" or "eco-friendly". While bio-based organic acids function identically to their synthetic counterparts, their sustainable production methods contribute to a lower environmental footprint, making them an attractive alternative for formulators seeking greener preservation solutions.
Recent noteworthy examples for this development are Benzoic Acid, which can be manufactured from the oxidation of natural benzaldehyde obtained from Cinnamomum cassia; (8) or Sorbic Acid, which is produced from biobased crotonaldehyde and ketene (9). In another category are those acids which have largely always been sustainably sourced, because it is the most cost-efficient method. This is true for Lactic Acid (10) and Citric Acid (11), which are both made in a fermentation process, or Levulinic Acid, which is produced from agricultural waste products such as sugar cane bagasse or corn cobs (12).
Conclusion
Organic acids have been a staple in cosmetic preservation for many years and will continue to help formulators protect their creations from contamination for many years to come. Their effectiveness hinges on formulation factors such as mainly the pH and formulation polarity. While they are not a one-size-fits-all solution, strategic formulation and pairing with complementary preservatives can enhance their antimicrobial performance. As the demand for clean beauty continues to grow, further research and innovative formulation techniques will be key to unlocking their full potential while ensuring consumer safety and product longevity.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
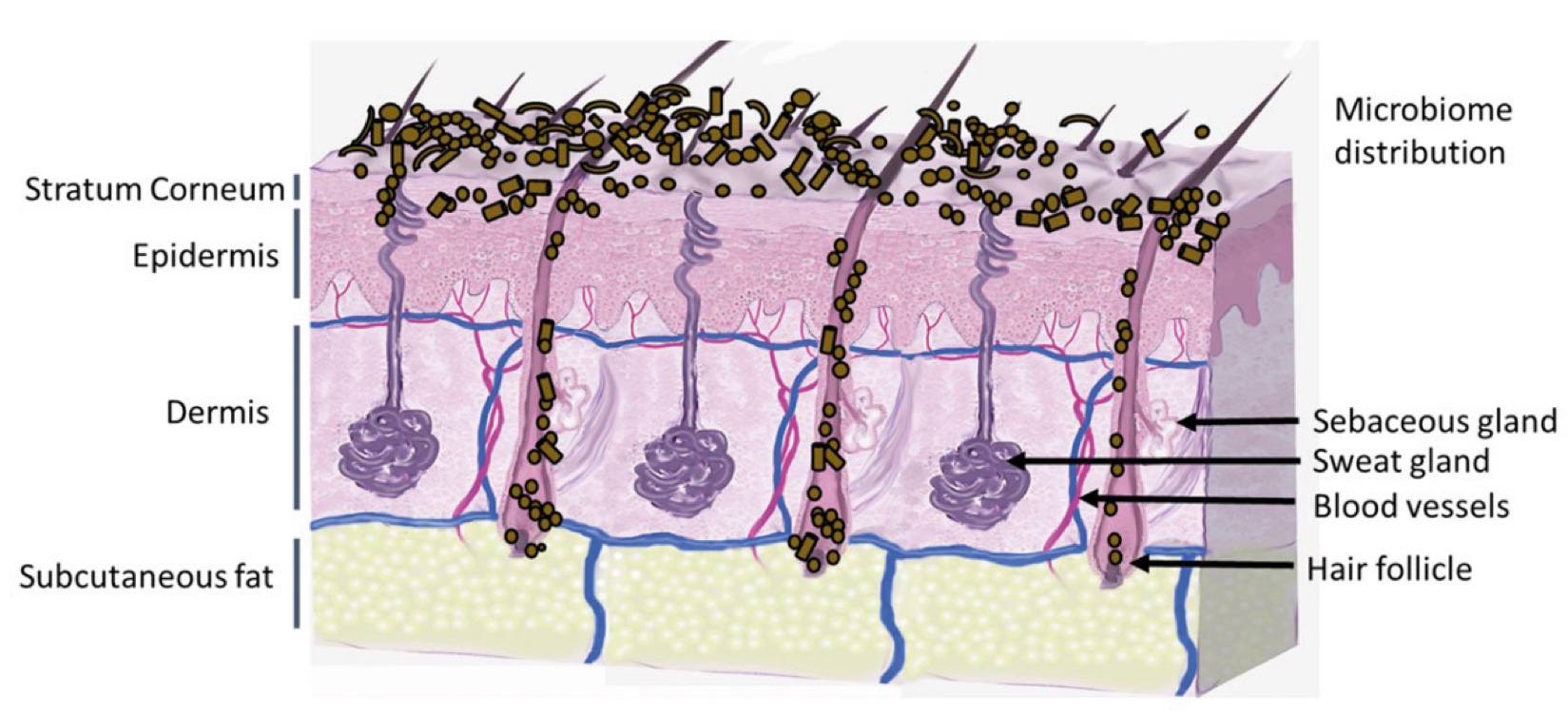
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
Zuo Y, Wang C, Zhan J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC−MS. Journal of Agricultural and Food Chemistry [Internet]. 2002 May 16;50(13):3789–94.
Brunner U. Some antifungal properties of sorbic acid extracted from berries of rowan(Sorbus aucupatia). Journal of Biological Education [Internet]. 1985 Mar 1;19(1):41–7.
Brul S, Coote P. Preservative agents in foods Mode of action and microbial resistance mechanisms. International Journal of Food Microbiology [Internet]. 1999 Sep 15;50(1–2):1–17.
Foster J. W., When protons attack: Microbial strategies of acid adaptation. Current Opinion in Microbiology, 1999, 2:170-i 74.
Henderson L. J., CONCERNING THE RELATIONSHIP BETWEEN THE STRENGTH OF ACIDS AND THEIR CAPACITY TO PRESERVE NEUTRALITY. Am. J. Physiol. 1908, 21 (2): 173–179.
Slater A.M., The IUPAC aqueous and non-aqueous experimental pKa data repositories of organic acids and bases. J Comput Aided Mol Des, 2014, 28, 1031–1034.
Michael Busch, Elisabet Ahlberg, Kari Laasonen, Universal Trends between Acid Dissociation Constants in Protic and Aprotic Solvents, Chem. Eur. J. 2022, 28 (59), 1-12.
Nguyen T. D., Conceptual Design for Synthesis of Benzaldehyde from Natural Cinnamomum Cassia Oil: Experiments and Simulation. Vietnam J. Sci. Technol. 2024, 62 (4), 635–647.
French Patent FR3028854A1
Ahmad A., Banat F., Taher H., A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environmental Technology & Innovation 2020, 20, 101138.
Milsom P.E., Organic Acids by Fermentation, especially Citric Acid. Food Biotechnology 1987, 1.
Rackemann D., William D., A review on the production of levulinic acid and furanics from sugars. International Sugar Journal, 2013, 115(1369), pp. 28-34.

