
Regulation
Skin care
peer-reviewed
Towards an EU Harmonised Definition of Nanomaterials: Implications for the Cosmetics Sector
BLANCA SUAREZ-MERINO
Director of Regulatory Affairs, Nanotechnology Industries Association, Brussels, Belgium
ABSTRACT: Nanotechnology is a transformative force in industries ranging from medicines to consumer goods, including cosmetics. In Europe, different regulations have different provisions for nanotechnologies, where definitions are not merely academic, but determine whether a product is regulated as containing nanomaterials or not. Understanding this connection is crucial for legal compliance, product development, and market success. This article explores the evolution of the EU’s definition of nanomaterials, its implications for cosmetics, and future challenges in characterising these materials should the EU recommendation for a definition of nanomaterials be adopted by the cosmetic regulation. A harmonised approach remains essential to facilitate innovation while ensuring consumer safety and regulatory compliance across Europe.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction
Nanotechnology has become ubiquitous, underpinning advances in diverse sectors from aerospace to healthcare. Its unique properties, such as small particle size and high surface area, enable functionalities that are otherwise unattainable at the macroscale.
Nanoscale titanium dioxide and zinc oxide are widely used in sunscreens as UV filters. At this scale, they block UV rays effectively while appearing transparent on the skin, avoiding the white residue of traditional sunscreens. Nanomaterials also enhance delivery of active ingredients, helping compounds like vitamins or antioxidants reach superficial skin layers more effectively, boosting the performance of creams and serums. Additionally, they improve product texture and finish. Silica nanoparticles, for example, scatter light to blur fine lines and imperfections, creating a smoother, more refined appearance in foundations and primers.
However, their unique properties also raise important questions about safety and consumer protection. For this reason, the European Union’s Cosmetics Regulation includes specific provisions for nanomaterials (1), however the lack of a harmonised definition initially led to uncertainty and fragmented compliance requirements across Member States.
In any case, the provisions in Regulation 1223/2009 aim to ensure that nanomaterials used in cosmetic products are both safe and transparently communicated to consumers.
A harmonised definition is also important for global trade. For cosmetics companies operating internationally, alignment with ISO standards and European definitions helps ensure that products can be sold in multiple markets without facing differing nanomaterial classifications and regulatory barriers.
For the cosmetics sector, the link between the definition of nanomaterials and regulation is both direct and critical. The definition determines whether manufacturers must notify authorities, label their products accordingly, and provide additional safety data. A misunderstanding of the definition can lead to regulatory non-compliance, product recalls, or barriers to market entry. Staying informed about evolving definitions and regulatory expectations is essential for ensuring product safety, protecting brand reputation, and maintaining access to the European and global markets.
Why Do We Need a Definition?
A nanomaterial definition serves as a regulatory gateway—triggering specific legal obligations if an ingredient qualifies. It is fundamental for consistent regulation and enforcement across EU policies. Without a harmonised framework, manufacturers face uncertainty, and regulators struggle to ensure safety and environmental protection.
Historically, the European Commission’s (EC) recommendation has served as a horizontal framework, applicable across diverse sectors. Several Member States, including France (2), Denmark (3), Sweden (4), Belgium (5), and Norway (6), have already implemented national measures relying on this EC definition.
It is important to note that the definition was never intended to distinguish hazardous from non-hazardous materials; rather, it focuses solely on size-based criteria to enable regulatory clarity.
The Evolution of Definitions
Over the years, various definitions have emerged globaly regarding nanomaterials (Figure 1):
- EU Cosmetic Regulation 1223/2009 defines a nanomaterial as an insoluble or biopersistent, intentionally manufactured material with external dimensions or internal structures between 1 nm and 100 nm.
- ISO/TS 80004-1:2010 defines nanomaterials as materials with any external dimension in the nanoscale, or having internal or surface structures in the nanoscale (1–100 nm). However, this definition focuses on particulate solid materials.
- EC Recommendation 2011/696/EU, updated in 2022, defines a nanomaterial as a natural, incidental, or manufactured material consisting of solid particles where 50% or more of the particles (in number-based size distribution) have one or more external dimensions in the 1–100 nm range.

Figure 1. Definition of nanomaterial across different organisation and sectors.
For the cosmetics sector, these distinctions are significant because only intentionally manufactured nanomaterials are regulated under EU Cosmetic Regulation 1223/2009, creating potential gaps for materials incidentally present or derived as by-products from other industries (Figure 2).

Figure 2. Differences among the EU recomendation for a definition of nanomaterials and the EU definition for nanomaterials in cosmetics.
In the European Union, if a product contains a material which falls under the definition depicted in Figure 2, further provisions need to be provided regarding notifications, safety assessment and labelling.
Besides the EU recommendation and the Cosmetic product definition, different EU countries have their own definitions (Table 1).
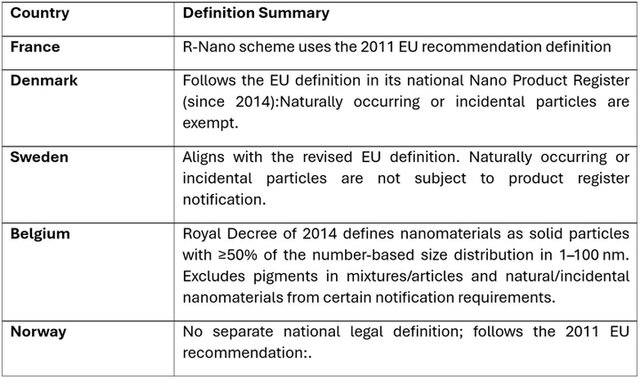
Table 1. Comparative overview of how selected European countries define 'nanomaterial' in national legislation or regulatory practice. While many definitions stem from the European Commission Recommendation (2011 or 2022), differences remain in interpretation and implementation.
Current regulatory landscape
The European Union’s Cosmetic Products Regulation (CPR) is currently undergoing a comprehensive evaluation what is called a “fitness check” of the CPR to assess whether the Regulation is still fit for purpose in the current European changing environment. Nanomaterials remain a significant area of attention in these ongoing updates.
The revision is not emerging in a vacuum. Over the past few years, the legislative landscape has been increasingly shaped by initiatives such as the European Green Deal (7), the Chemicals Strategy for Sustainability (8), and broader political goals like decarbonisation and digital transformation.
However, updating the definition alone is not sufficient. Industry stakeholders stress the need for a clear transition mechanism to manage positive lists of nanomaterials permitted in cosmetics. Without this, sudden regulatory shifts could disrupt the market, especially for existing products. A targeted amendment of the CPR would allow alignment with science and international standards while avoiding major disruptions for manufacturers and importer.
The timeline for the revision starts with the results of the evaluation expected to be published by mid-2026, followed by an Inception Impact Assessment by the end of that year. A legislative proposal for revising the CPR is anticipated by the end of 2027, with the legislative procedure potentially concluding in 2028. This suggests that a revised CPR could be published in early 2029 (9).
Challenges in Characterising Nanomaterials
Characterising nanomaterials under the EU 2022 definition presents several technical challenges, especially regarding particle size, shape, and structure. Nanoparticles are often irregular, existing as single particles, aggregates, or agglomerates, raising questions about which dimensions to measure. The EU definition relies on direct physical measurements (e.g., Feret diameter, maximum inscribed circle) rather than indirect equivalents, which can misclassify irregular particles. For elongated or plate-like shapes, cross-sectional dimensions or thickness are key.
Distinguishing between aggregates (strongly bound) and agglomerates (loosely held) is also critical, as constituent particle dimensions are generally preferred for classification—though sometimes only the aggregate can be measured. The JRC requires all identifiable particles to be counted in number-based size distributions, which better reflect the presence of nanoscale particles than mass-based methods.
The definition applies only to solid particles, excluding liquids, gases, and single molecules. Additional complexity arises from nanomaterials' nonlinear, fractal, and dynamic behavior, which challenges traditional models and leads to batch variability.
In cosmetic matrices, nanomaterial detection is further complicated by emulsions, encapsulation, and matrix interference. Advanced imaging techniques like TEM are often required but remain labor-intensive. Overall, accurate classification demands robust, context-specific methods and ongoing scientific refinement.
Regulatory Implications for Cosmetics
Under the European Union’s Cosmetics Regulation (EC) No 1223/2009, if an ingredient in a formulation is defined as a nanomaterial, the manufacturer must follow a series of obligations. Conversely, if the ingredient does not meet this definition, these requirements do not apply. This is why it is so important for companies to determine whether a substance truly qualifies as a nanomaterial under the regulation.
Under EU Cosmetic Regulation 1223/2009, only intentionally produced nanomaterials are regulated, excluding incidental or natural particles. Consequently, materials such as cellulose derived from other industries might not be considered nanomaterials if they have not been deliberately engineered to nanoscale dimensions.
In contrast, the broader EU 2022 update encompasses all naturally occurring, incidental, and intentionally produced nanomaterials, applying stricter criteria for classification and potentially widening the regulatory net.
Despite these differences, a harmonised definition remains critical to enable safe innovation in cosmetic formulations while ensuring consistent regulatory compliance and consumer protection (Table 2).
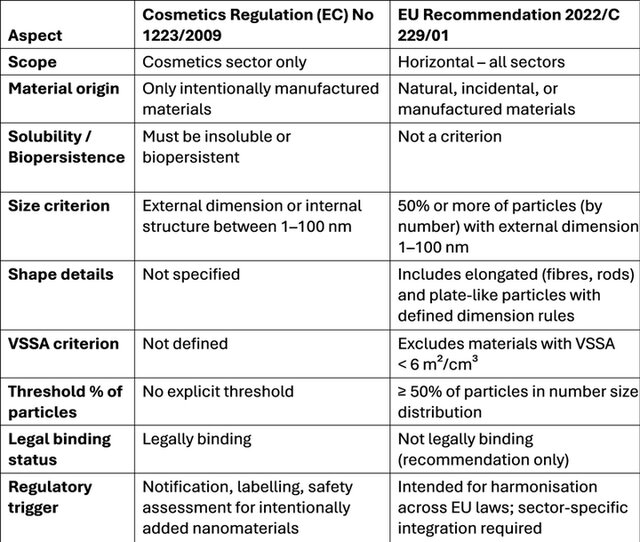
Table 2. Comparison of Nanomaterial Definitions: Cosmetics Regulation vs EU Recommendation 2022.
Changes discussed in Table 1 have profound implications for the classification of various cosmetic ingredients. In essence, materials currently regulated as nanomaterials under the CPR may no longer fall under this category if the revised definition is adopted, while conversely, other substances not previously considered nanomaterials may meet the criteria. A few examples are provided below:
Ingredients Potentially No Longer Classified as Nanomaterials
Titanium Dioxide (TiO₂)
Titanium dioxide is widely used in sunscreens and cosmetic formulations as a UV filter. Under the current CPR definition, TiO₂ produced with primary particles in the nanoscale range is regulated as a nanomaterial. However, many commercial grades of nano-TiO₂ are heavily aggregated or surface-coated, which can result in a specific surface area below the 6 m²/cm³ threshold. Should the revised definition be adopted, certain aggregated or coated forms of TiO₂ may no longer qualify as nanomaterials, provided their number-based size distribution reflects a majority of particles exceeding 100 nm and their specific surface area remains low (European Commission JRC, 2022).
Silica (SiO₂)
Similarly, certain forms of silica, such as fumed silica used as rheology modifiers in cosmetic creams, possess primary particle sizes within the nanoscale. However, these materials often exist as large, fused aggregates with relatively low specific surface areas. If their specific surface area is below 6 m²/cm³, they may fall outside the revised nanomaterial definition, even though they have historically been treated as nanomaterials under the CPR.
Ingredients Potentially Newly Classified as Nanomaterials
Carbon Black
Carbon black, used as a pigment in cosmetics such as mascaras and eyeliners, has historically been considered a non-nanomaterial due to its aggregated state. However, primary particles frequently measure 30–50 nm in diameter. Under the revised definition, if particle counting techniques reveal that >50% of the particles in number-based distributions are nanosized and the material’s specific surface area exceeds 6 m²/cm³, carbon black could newly be classified as a nanomaterial, thereby triggering additional regulatory obligations.
Certain Bulk Pigments and Additives
Similarly, some pigments and additives not previously regulated as nanomaterials may become subject to nanomaterial requirements if advanced characterisation techniques detect a high proportion of nanoscale particles. This is particularly relevant for materials with complex manufacturing processes, where micron-sized aggregates conceal nanoscale primary particles that would only be revealed through techniques such as electron microscopy or field-flow fractionation (10).
Ingredients Remaining Excluded
Nanoemulsions and Labile Nanostructures
It is noteworthy that the revised definition explicitly targets solid particles and excludes non-solid structures. Thus, nanoemulsions, liposomes, micelles, and other labile self-assembled systems would remain outside the nanomaterial definition under both the current CPR and the 2022 Recommendation. These systems disintegrate upon application and lack the solid, persistent character associated with nanomaterials of regulatory concern.
Conclusion
Nanotechnology is reshaping the cosmetics industry, enhancing product performance and consumer benefits. The EU has updated its nanomaterial definition through Commission Recommendation 2022/C 229/01, which, if taken up by the CPR may lead to reclassification of common ingredients like titanium dioxide, silica, or carbon black, prompting new obligations for manufacturers. Dynamic systems, where particles aggregate or disassemble, further complicate compliance. Ongoing regulatory updates and scientific research are vital to ensure effective implementation and to help industry navigate evolving EU requirements for nanomaterials in cosmetics.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
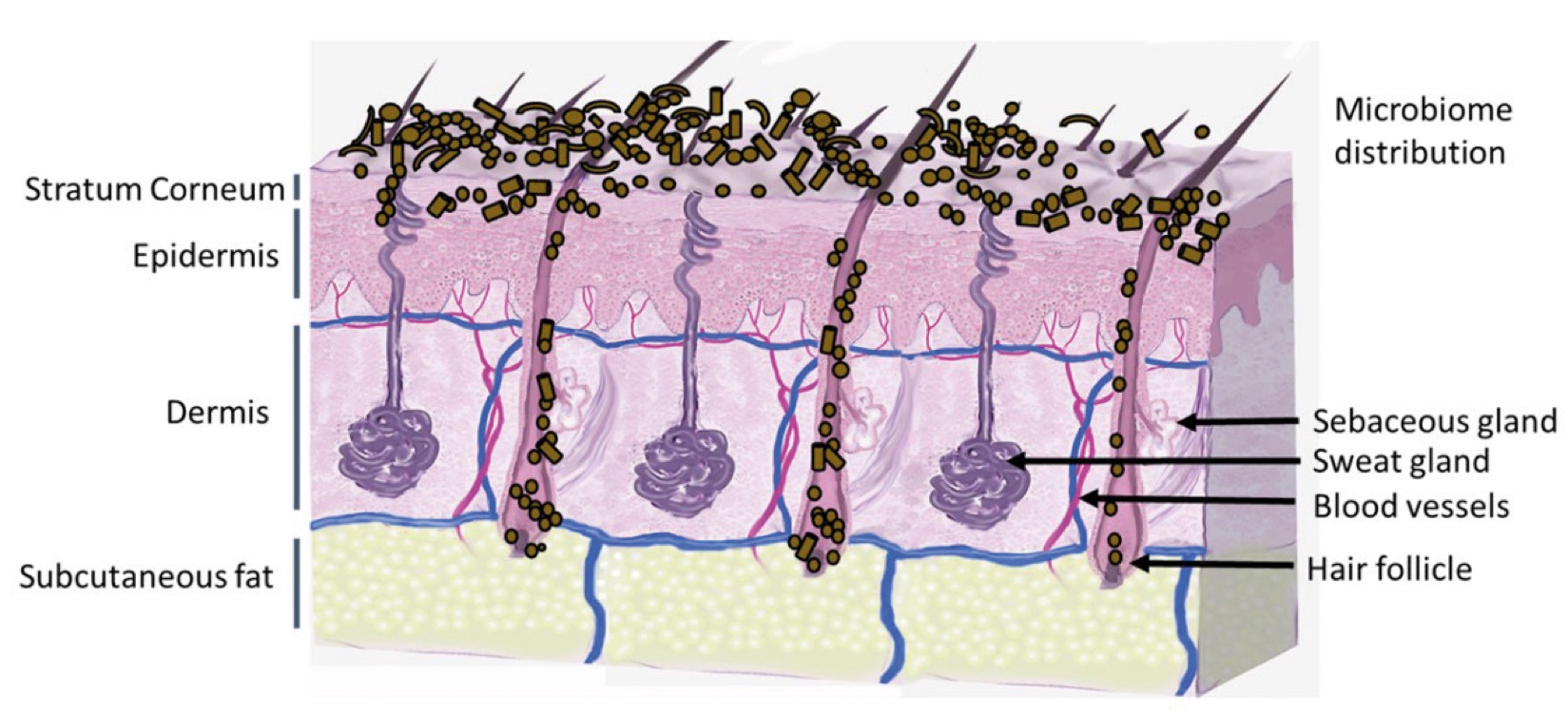
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
1. (EC) No 1223/. European Parliament and Council. 2009. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. OJ 342. 2009;59-209. https://eur-lex.europa.eu/eli/reg/2009/1223/oj
2. Décret n°. 2012‑232. France. 2012. Decret du 17 février 2012 portant définition des substances à l’état nanoparticulaire pour le dispositif de déclaration “R‑Nano”. Journal Officiel de la République Française. 2012; https://www.legifrance.gouv.fr/eli/decret/2012/2/17/DEVP1123456D/jo/texte
3. Order No. 644/2014. Denmark. 2014. Order on a Register of Mixtures and Articles Containing Nanomaterials, Statutory Order No. 644/2014 (18 June 2014). 2014; https://www.bing.com/ck/a?!&&p=e46a90cbe744af5da5b0a3adb95e027485aa832ecbc51fbf4979cc17501a0287JmltdHM9MTc1NjA4MDAwMA&ptn=3&ver=2&hsh=4&fclid=22b76fce-c0e5-6ac7-304d-799dc1186ba7&psq=Bekendtg%c3%b8relse+om+register+over+blandinger+og+varer%2c+der+indeholder+nanomaterialer+samt+producenter+og+import%c3%b8rers+indberetningspligt+til+registeret%22&u=a1aHR0cHM6Ly93d3cucmV0c2luZm9ybWF0aW9uLmRrL2VsaS9sdGEvMjAxNC82NDQvcGRmIzp-OnRleHQ9QmVrZW5kdGclQzMlQjhyZWxzZW4lMjBoYXIlMjB0aWwlMjBmb3JtJUMzJUE1bCUyMGF0JTIwb3ByZXR0ZSUyMGV0JTIwcmVnaXN0ZXIsdmFyZXJuZSUyMG9nJTIwZGUlMjBuYW5vbWF0ZXJpYWxlciUyQyUyMGRlJTIwaW5kZWhvbC1kZXIlMkMlMjB0aWwlMjBuYW5vcHJvZHVrdHJlZ2lzdGVyZXQu&ntb=1
4. KIFS 2017:7. Swedish Chemicals Agency. 2017. KIFS 2017:7 – Regulations Amending the Products Register (Chapter 3: Nanomaterials). December 19, 2017; effective January 1, 2018. 2018; https://www.kemi.se/en/rules-and-regulations/agency-regulations-kifs
5. Belgium Decret. Belgium. 2014. Arrêté royal relatif à la mise sur le marché des substances manufacturées à l’état nanoparticulaire (27 May 2014). Belg Off J. 2014; https://vlex.be/vid/sur-des-substances-nanoparticulaire-532325634
6. NANO-box. Norwegian Environment Agency. 2013. Amendment to the annual update of the Norwegian Product Register: Implementation of nanomaterial notification (“NANO-box”) requirements. Oslo: Norwegian Environment Agency, January 9. Based on EC Recommendation 2011/696/EU. 2013; https://www.lawbc.com/norway-requires-information-on-norwegian-product-register-chemicals-in-nanoform/
7. Green Deal Europe [Internet]. 2019. Available from: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en
8. European Commission. Directorate General for Environment. Chemicals strategy for sustainability: towards a toxic free environment. [Internet]. LU: Publications Office; 2020 [cited 2025 Jul 13]. Available from: https://data.europa.eu/doi/10.2779/221541
9. (EC No 1223/2009). European Commission. Evaluation (“Fitness Check”) and Targeted Revision of the Cosmetic Products Regulation (EC No 1223/2009). Inception Impact Assessment, Call for Evidence (February 21–March 21, 2025), and Public Consultation (Q2 2025–July 28, 2025). Brussels: European Commission, 2025. 2025;
10. European Commission. Joint Research Centre. Guidance on the implementation of the Commission Recommendation 2022/C 229/01 on the definition of nanomaterial. [Internet]. LU: Publications Office; 2023 [cited 2025 Jul 12]. Available from: https://data.europa.eu/doi/10.2760/143118.
