
Regulation
Skin care
peer-reviewed
The EU Packaging and Packaging Waste Regulation:
A cosmetic industry perspective
AMANDA ISOM
Regulatory Affairs Director, Bloom Regulatory Ltd, London, England
ABSTRACT: Building on the 1994 framework on packaging and packaging waste, the EU’s new Packaging and Packaging Waste Regulation (PPWR), effective August 2026, marks a significant shift in packaging design and management. It introduces stricter rules on design and recyclability. The PPWR supports the EU Green Deal, aiming for safety for consumers, less waste, and more recycling. Key aspects include minimising ‘Substances of Concern’, ensuring all packaging is recyclable by 2030, and mandating minimum recycled content in plastic packaging. The Regulation also introduces a harmonised waste sorting label and requires a Declaration of Conformity and Technical Dossier to prove compliance. In this article, we’ll look at some of the key concepts and obligations relevant to the cosmetics industry, including: substances of concern, recyclability, recycled content, packaging minimisation, waste sorting labels, and the technical dossier.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”

Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction
On 22 January 2025, the EU adopted the new Packaging and Packaging Waste Regulation (PPWR). This regulation will come into effect on 12 August 2026, marking a significant change in how packaging is designed, managed, and regulated across the EU.
Since 1994, the EU and Great Britain have followed a packaging and packaging waste framework designed to:
- Reduce the amount of packaging used
- Cut down on packaging waste
- Limit hazardous substances in packaging
This framework, often referred to by its ‘essential requirements’, required businesses to minimise packaging, design it for recyclability, and eliminate heavy metals and other harmful substances.
The new PPWR builds on this foundation rather than replacing it. Key updates include:
- It is now a regulation rather than a directive, ensuring consistent application across the EU
- It introduces stricter rules for packaging design, recyclability, and reuse
- It sets out clearer obligations for producers, backed by stronger enforcement mechanisms
The PPWR is part of the EU’s broader Green Deal and circular economy goals, aiming to reduce waste, increase recycling, and make better use of resources.
In this article, we’ll look at some of the key concepts and obligations relevant to the cosmetics industry, including: substances of concern, recyclability, recycled content, packaging minimisation, waste sorting labels, and the technical dossier.
Substances of Concern in Packaging
Defined by the Ecodesign for Sustainable Products Regulation (ESPR), the concept of ‘Substances of Concern’ (SoC) will play an important role in many legislative frameworks published under the Green Deal. With regards PPWR:
- Packaging should be manufactured to minimise the presence and concentration of substances of concern (by Aug 2026).
- The sum of concentrations of lead, cadmium, mercury, and hexavalent chromium in packaging or components should not exceed 100 mg/kg
Within ESPR, SoC are defined as:
- substances of very high concern (SVHC) under EU REACH;
- substances falling under specific hazard categories of the CLP Regulation, such as carcinogenic, mutagenic or reprotoxic (CMR), persistent, bioaccumulative and toxic (PBT), very persistent and very bioaccumulative (vPvB), and specific target organ toxicity (STOT);
- persistent organic pollutants (POPs);
- and any substance that negatively impacts the reuse or recycling of packaging.
Whilst minimisation and substitution are the primary goal, it may not always be possible to completely eradicate all SoC from packaging. Packaging containing substances of concern will have to be marked using standardised, open, digital technologies in the future as part of the digital product passport and transparency principles.
Recyclable Packaging
One of the central pillars of the PPWR is the requirement for all packaging to be recyclable by 2030. This will introduce clear, enforceable obligations for businesses across the packaging supply chain as well as increase consumer expectation in this area.
Within the PPWR, obligations will be introduced to ensure that packaging will not only be theoretically recyclable but that it can also be recycled on a practical basis. This will mean that packaging must be both designed for recycling and also be able to be part of an effective collection, sorting, and recycling system. Packaging that combines the two criteria means it is considered to be ‘recyclable’.
To make this requirement achievable for industry, each criterion will need to be supported by additional legislation to clarify what 'Design for Recycling' entails and what qualifies as 'recycled at scale'.
The European Commission, Parliament, and Council acknowledge that such significant changes to packaging cannot happen overnight, and that the infrastructure needed to recycle all materials at scale is not yet fully in place. As a result, the final PPWR text includes phased deadlines and transitional measures to support progress toward its long-term goals.
The first key milestone comes in 2030, when only packaging rated A to C will be allowed on the market. By 2038, the requirements become stricter, permitting only packaging that meets at least a grade B standard.
Recycled Content
The PPWR introduces mandatory requirements for minimum levels of recycled plastic content in packaging, including for cosmetic products. These new rules aim to reduce reliance on virgin plastics and promote the use of post-consumer recycled (PCR) materials. However, while the intent is environmentally driven, the implementation raises complex challenges for industries where product safety, stability, and consumer protection are key.
From Jan 2030 and Jan 2040, plastic packaging should contain a minimum percentage of recycled content recovered from post-consumer plastic waste, calculated as an average per manufacturing plant and year.

The new PPWR requirements represent a significant step toward more sustainable packaging practices in the cosmetics industry. However, meeting these recycled content targets poses technical and supply chain challenges. The limited availability of high-quality recyclates means the industry must look to adapt and adopt new risk assessment strategies. Initiatives like CosPaTox (1) offer valuable tools to bridge this gap, providing structured guidance on how to evaluate and safely integrate PCR materials. As regulatory timelines approach, proactive collaboration between stakeholders will be essential to ensure that both sustainability and product safety are maintained.
Packaging Minimisation
Both the existing Packaging and Packaging Waste Directive (94/62/EC, or PPWD) and the Packaging and Packaging Waste Regulation (EU 2025/40, or PPWR) require companies to reconsider how they package products. One key focus is packaging minimisation.
Packaging minimisation is all about eliminating unnecessary packaging, especially packaging that exaggerates the size of a product to boost appearance on shelf. In essence, packaging must be designed and manufactured so that its volume and weight are no more than what is needed to ensure product safety, hygiene, and consumer acceptance. That means no oversized or deceptive boxes, unnecessary inserts, or decorative components that don’t serve a functional purpose.
Packaging minimisation plays a key role in the ‘waste hierarchy’ set out in the EU Waste Framework Directive (Directive 2008/98/EC). This hierarchy ranks waste management options from most to least environmentally friendly, starting with prevention and ending with disposal, e.g. landfill.
Minimising packaging is part of the most favoured prevention stage. It focuses on actions taken before a material or product becomes waste, so reducing:
- the amount of waste generated;
- the environmental and health impacts of waste; and
- the presence of harmful substances in materials and products.
Under PPWR, this means:
- Packaging must be designed to minimise weight and volume.
- False or misleading packaging characteristics like double walls or false bottoms will be banned.
- Transport and e-commerce packaging must not exceed an empty space ratio of 50%.
- Single-use plastic packaging for cosmetics, hygiene, and toiletry products used in the accommodation sector will be prohibited.
In practice, reducing packaging size, without compromising safety or usability, requires a balanced approach across all components of a product, along with efforts to minimise hazardous substances. Many companies demonstrate compliance by developing a Packaging Minimisation Dossier, which documents design choices, justifications, and supporting evidence. This is often supported by a broader packaging strategy that explains how minimisation decisions are made and how environmental design principles are applied.
Sorting Label
Several EU Member States, including France, Italy, Spain, and Portugal, already define sorting instructions that must be supplied to consumers but the requirements are diverse. The PPWR will introduce a new harmonised label that will standardise packaging disposal information across all Member States, whilst maintaining that packaging provides indication of the material(s) used and the disposal route (e.g. plastic recycling bin)
Not part of the PPWR when published, the European Commission is tasked with developing the technical details of the labelling system through implementing acts, which will define how the label should appear, how it should be used, and when exactly businesses must begin applying it to packaging.
Earlier this year, the European Commission launched a targeted consultation to gather feedback from consumers, industry stakeholders, and local authorities on the design of the harmonised label. The draft guidelines shared as part of this consultation provided a proposed design for use on consumer packaging and the corresponding waste receptacles, featuring a packaging icon, such as plastic bottle, on coloured background and text description of the waste stream e.g. plastic. We will need to wait to see if this proposal moves forward or whether new formats will be proposed in the coming months.
Technical Dossier
Once all requirements of the PPWR detailed in Articles 5 to 12 are met, compliance must be demonstrated via a Declaration of Conformity (DoC) and supporting Technical Dossier. Just like the Product Information File for a cosmetic product, this is a structured collection of evidence that demonstrates that compliance has been achieved.
Many of the details of ‘how’ to comply with requirements of the PPWR are yet to be decided and so the exact content of the technical dossier cannot be fully determined at this time (e.g. choice of assessments and methodologies). However, as deadlines approach and more detail becomes available over aspects such as determining recyclability, this dossier will need to be populated and updated to document and demonstrate design choices, materials used, recyclability assessment, and other relevant test results.
Conclusion
It is important to remember that some of the provisions of the PPWR already exist under the current PPWD and compliance is currently required in areas such as:
- Minimisation of noxious and hazardous substances, in particular the levels of lead, cadmium, mercury, and hexavalent chromium;
- Minimisation of packaging and, in particular, replacing items such as false bottoms and deceptive thick walled packaging, and removing unnecessary layers.
- Preparing packaging minimisation information for each product.
Although 2030 may seem far off, meeting requirements for recyclability and recycled content can take considerable time, especially when balancing other factors like safety from hazardous substances, packaging minimisation, and managing existing stock.
Some definitions still need clarification through additional Implementing Acts or Regulations. However, we believe companies should begin assessing their current packaging portfolio now to identify priority areas for change.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
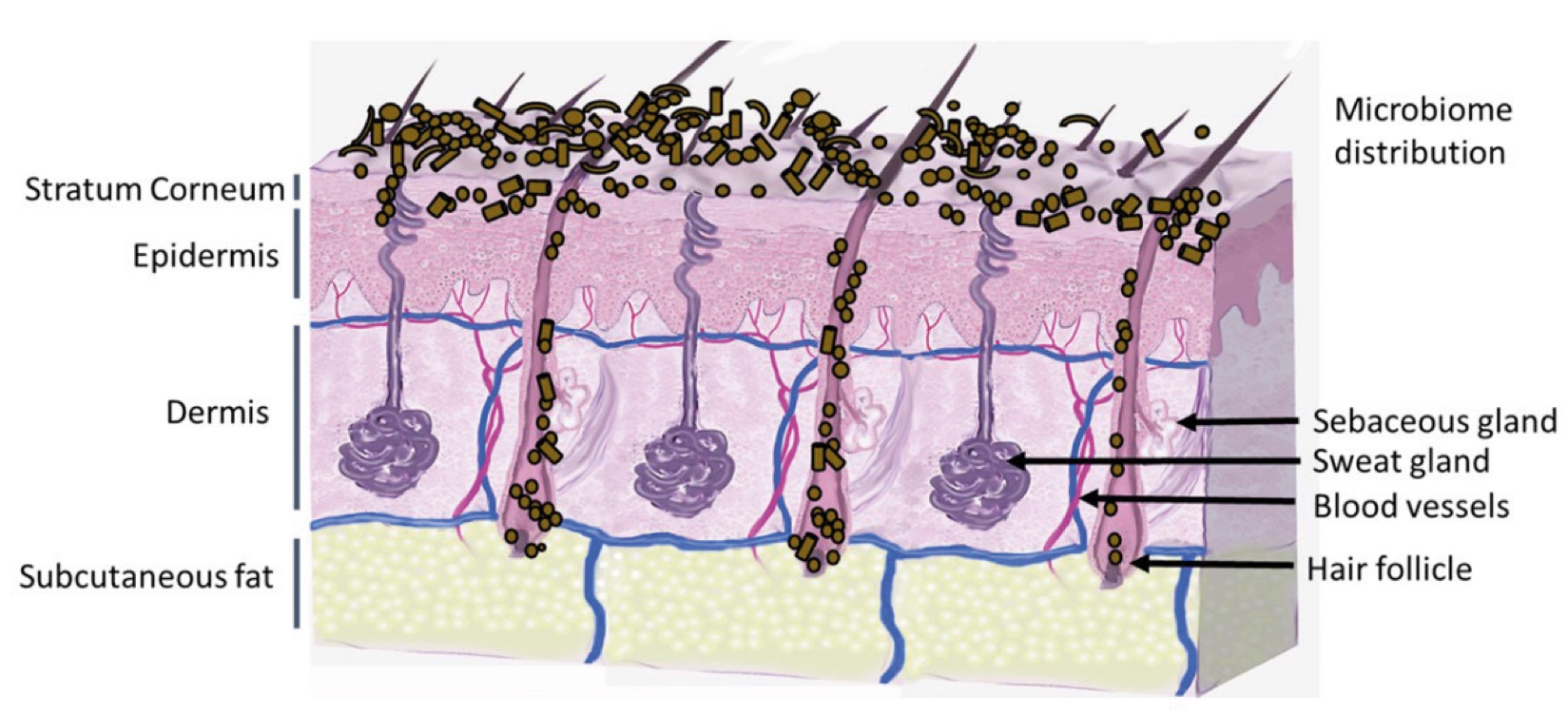
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- CosPaTox Consortium. Safety assessment guidelines for post-consumer recycled plastics (PCRs) for cosmetics and detergents packaging [Internet]: CosPaTox; [cited 2025 Jul 21]. Available from: https://cospatox.com/publication/
