
Regulation
Skin care
peer-reviewed
The EU simplification process – easing regulatory burden while ensuring the highest standard of safety
John Chave
Director General, Cosmetics Europe, Brussels
ABSTRACT: This article examines the European Commission Omnibus VI proposal and highlights the positive impact the changes proposed will have on the cosmetics and personal care industry if adopted. Cosmetics Europe explains how these changes will preserve the highest safety standards while introducing pragmatic clarifications to the Cosmetic Products Regulation (CPR). The proposed changes will allow the European cosmetics industry to continue using safe and effective ingredients in cosmetic products, driving innovation and consumer choice, ultimately strengthening the global competitiveness of the sector.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
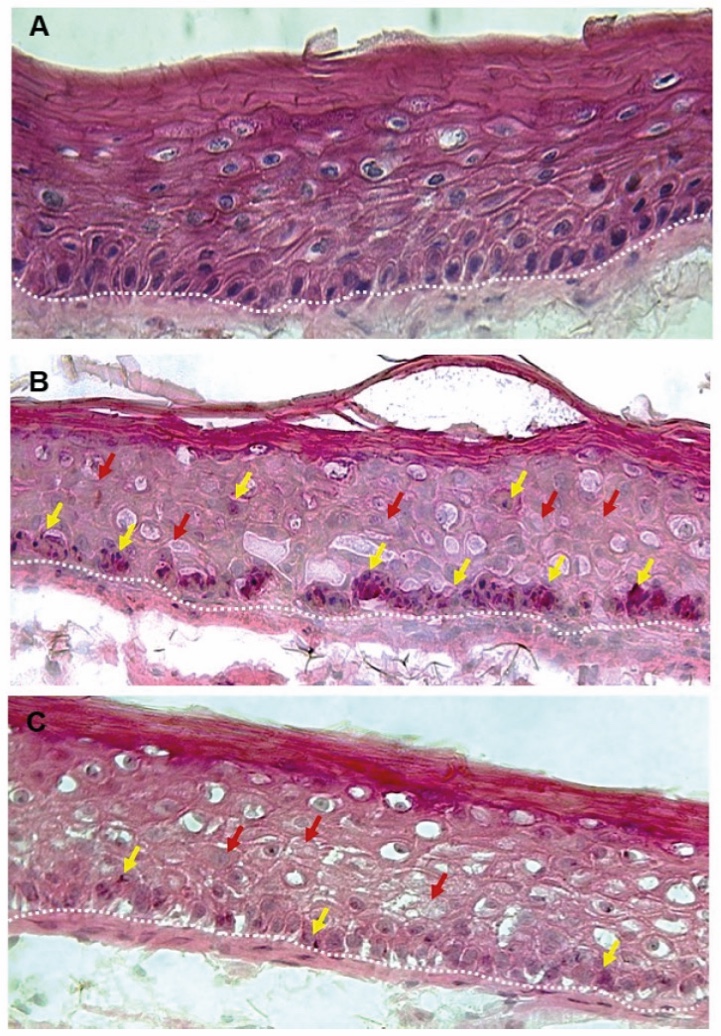
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction
The European cosmetics and personal care industry is a dynamic, innovative and export-oriented sector. While many multinational companies are household names, it is less well known that there is a huge and diverse constituency of small and medium-size enterprises (SMEs), more than 9600, in Europe, contributing significantly to the sector’s dynamism and export success – our industry had a trade surplus of nearly 21 million in 2024 (1).
However, Europe, once the largest market for cosmetics and personal care products, has fallen second behind the US in recent years. During this time an increase in the number of regulations impacting our industry initiated by the EU has created challenges for our companies. The challenge of burdensome regulation and its impact on Europe’s competitiveness in general was recently highlighted by former Italian Prime Minister and head of the European Central Bank, Mario Draghi, in his report on European competitiveness (2).
In particular, we have witnessed increased regulatory focus on ingredients used in cosmetics. For instance, the EU’s flagship Green Deal project, launched in 2020, which aimed to introduce a more precautionary approach to chemicals in general, including the introduction of new, restrictive criteria (3).
While the industry does not of course oppose regulation, and does not compromise on safety, disproportionate regulation (that is regulation that goes beyond what is necessary to achieve safety) may have negative implications for competitiveness, by for example imposing unnecessary costs, diverting resources from innovation, or reducing product functionality. This impact is felt disproportionality by SMEs, which the EU itself recognises are an important engine for growth (4).
Rising concerns relating to the relative decline of the European economy, as highlighted in the Draghi report, as well as the evolving geopolitical context, led to a commitment by the European Commission president, Ms Von der Leyen, to prioritise competitiveness in her second term, which began in November 2024.
In January 2025, she launched a Competitiveness Compass highlighting eight areas of EU policy action which address competitiveness related issues (5). One area – ‘simplification’ explicitly aims to tackle what Ms Von Der Leyen refers to as ‘red tape ’or excessive regularity burden, while remaining committed to sustainability goals.
Simplification addresses specific regulatory burdens across a number of sectoral and horizontal (applying to many sectors) regulations. The vehicles for Simplification are Omnibus measures, so called because they address multiple EU Directives or Regulations in a single statutory instrument. One such Omnibus, Omnibus VI, published in July 2025 addresses chemical legislation (6).
CMRs under the CLP and CPR
A major concern of the European cosmetics industry has been the interplay between Classification, Labelling and Packaging (CLP) Regulation and the Cosmetics Products Regulation (CPR).
Under the CLP, chemicals can be classified as hazardous within a range of specified hazard classes. One such hazard class is carcinogenic, mutagenic and reprotoxic (CMR). Classification as hazardous does not in itself constitute a ban on the use of such substances.
However, responding to concerns about the possible risk to consumers of such ingredients, the CPR provides that unless a derogation is granted in accordance with strict criteria, then under Article 15 of the CPR, a classified CMR category 1 is automatically banned for use in cosmetics. It is to be noted here that classification under CLP is on a hazard basis – that is, irrespective of consumer exposure, while for chemicals falling outside the CLP framework, the CPR provides for assessment on a risk/exposure basis, overseen by the EU’s Scientific Committee on Consumer Safety (SCCS).
Such bans have significant consequences for the industry and for consumers, including loss of valued ingredients, unnecessary reformulations, and removal of safe products from the market.
The issue has become more acute in recent years, due to a significant increase in CMR classifications or intentions to classify cosmetic ingredients. Recent examples include P-Cymene, heliotropin, Sodium Fluoride and Ethanol. This development has created a significant degree of concern within the cosmetics industry, because while the industry is confident in the safety of such substances in cosmetic uses, the regulatory framework under Article 15, in particular the unclear derogation criteria and the lack of a clear definition of what constitutes a suitable alternative, constrain the industry’s scope for successfully demonstrating that use in cosmetic should continue to be permitted. In the worst case, this threatens perfectly safe ingredients with removal from the market. In the past 12 years, only two substances* were considered for a derogation, and both requests were denied despite the SCCS concluding that they could be safely used in certain types of cosmetics (7, 8, 9, 10). This then would seem to be a case where ‘simplification ‘might be relevant, by facilitating the application of derogations, provided of course that safety is in no way compromised.
The Omnibus VI on chemicals addresses this question, by among other things refining the derogation criteria and introducing clearer deadlines for the submission of evidence that a derogation is appropriate.
Derogation Criteria
Under the current rules, to make use of a derogation, the industry must show that the CMR substances is approved for safe use in food. Additionally, the industry must demonstrate that there are ‘no suitable alternatives’ to the substance in question.
These aspects of the criteria are problematic in two respects, First, clearly ingestion in food is a different form of exposure compared to dermal application in cosmetics. Second, the concept of suitable alternatives is vague and open to interpretation, functionally inferior or prohibitively expensive materials could possibly be considered ‘suitable’.
The Omnibus VI proposes to abolish the food criterion entirely. With regard to suitable alternatives, the Omnibus VI text proposes for a range of aspects to be considered in the criteria for assessing alternatives including human health, environment, efficacy, feasibility, availability and economic aspects. Economic aspects take into account the practical feasibility and availability of using alternatives at scale.
Crucially, the Omnibus VI maintains the provision that requires the SCCS to confirm the safety of the ingredient for use in cosmetics under the relevant exposure scenario.
Safety
Concerns have been expressed by some stakeholders that changes proposed by Omnibus VI may lead to consumers being placed at risk. Given the significant level of public sensitivity around chemicals safety, and perhaps carcinogenicity in particular, this was predictable. But such concerns rely on a fear of intrinsic hazard properties, rather than the scientific analysis of exposure which is at the heart of modern toxicology and the guiding principle of the EU’s own consumer safety body, the SCCS.
The cosmetics industry believes that the approach in the Omnibus VI will have no negative consequences for safety but will in fact facilitate the continuing use of substances which have been demonstrated to be safe in cosmetics, notwithstanding a CLP hazard classification.
To add further context to the issue, it is worth noting that the number of CMR-classified substances actually used in cosmetics is extremely limited, and when such substances are present, their use is strictly regulated and demonstrably safe. The cosmetics industry relies on alternatives wherever feasible, and in the vast majority of cases, this is exactly what happens. However, there remains a handful of situations where the ingredient can be used safely and no suitable alternative exists – only those ingredients are the ones we want to keep using in our cosmetics.
Other measures
The Omnibus VI also includes other notable simplification measures, including the removal of the need to pre-notify nanomaterials under Article 16 of the CPR, which addresses duplication. Indeed, as the CPR stands currently the notification of products containing nanomaterials is required under both Article 13 and Article 16. The removal of pre-notifying nanomaterials under Article 16 will helps reduce administrative burden for the industry. Additionally, the measure establishes a procedure for the inclusion on Colorants, Preservatives and UV filters in the CPR annexes, addressing a long-held frustration of the industry that innovative substances, which cannot be used until included in the annexes, were potentially subject to being caught in a bureaucratic limbo.
Next steps
The version of the Omnibus VI proposed by the European Commission is only the beginning. The measure is now subject to scrutiny and possible amendment both by the EU Members States and the European Parliament. As such the Omnibus is something of a test case of the ability of the EU as a whole to simplify regulations and promote competitiveness. It is to be hoped that the fact that the measure in no way dilutes safety protection is fully understood by all stakeholders.
In conclusion, the proposal if adopted will ultimately support competitiveness by helping to avoid unnecessary reformulation, promoting innovation based on a wide ingredients palette, and maintaining ingredients which help the industry meet the needs and demands of its consumers.
As such, cosmetic ingredients and products currently meet and will continue to meet the EU’s high safety standards under the CPR, even with the changes proposed in the Omnibus VI on Chemicals.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
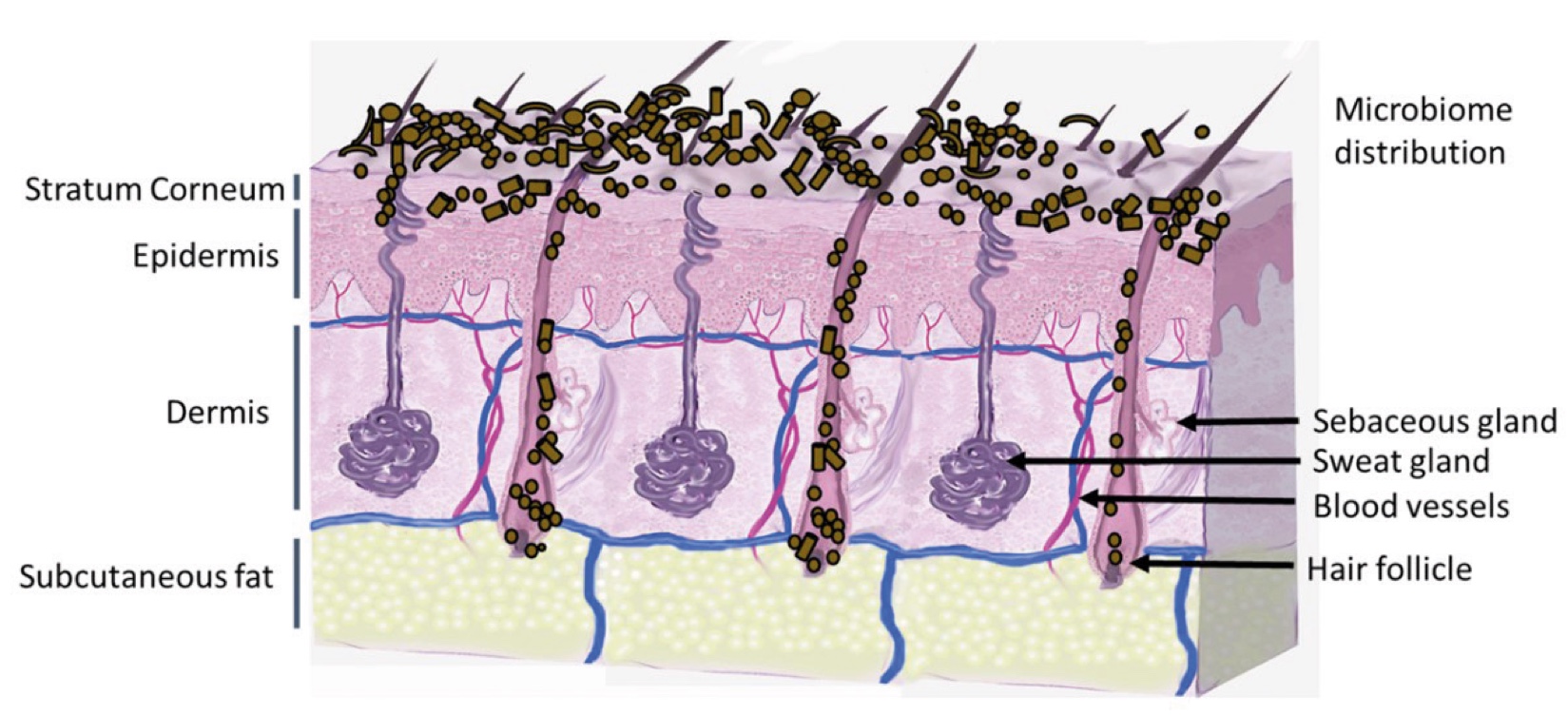
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
* The two substances are Formaldehyde and Zinc Pyrithione
- Cosmetics Europe Market Performance Report 2024. https://cosmeticseurope.eu/resources/ce-statistics-2024/
- Draghi M. The future of European competitiveness: A competitiveness strategy for Europe. 2024. https://commission.europa.eu/topics/eu-competitiveness/draghi-report_en
- European Commission. Chemicals Strategy for Sustainability – Towards a toxic-free environment. 2020. https://environment.ec.europa.eu/strategy/chemicals-strategy_en
- European Commission. Press Release: An EU Compass to regain competitiveness and secure sustainable prosperity.2025. IP_25_339_EN.pdf
- European Commission. Competitiveness Compass 2025. https://commission.europa.eu/topics/eu-competitiveness/competitiveness-compass_en
- European Commission. Proposal for a regulation – Simplification of certain requirements and procedures for chemical products 2025.https://single-market-economy.ec.europa.eu/publications/simplification-certain-requirements-and-procedures-chemical-products_en
- Commission Regulation (EU) 2019/831 of 22 May 2019 amending Annexes II, III and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Official Journal European Union. 2019 May. http://data.europa.eu/eli/reg/2019/831/oj
- Scientific Committee on Consumer Safety (SCCS). Opinion on the safety of the use of formaldehyde in nail hardeners. European Commission; 2014 November. https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_164.pdf
- Commission Regulation (EU) 2021/1902 of 29 October 2021 amending Annexes II, III and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards the use in cosmetic products of certain substances classified as carcinogenic, mutagenic or toxic for reproduction. Official Journal European Union. 2021 November. https://eur-lex.europa.eu/eli/reg/2021/1902/oj
- Scientific Committee on Consumer Safety (SCCS). Opinion on Zinc Pyrithione (ZPT). European Commission; 2020 March. https://health.ec.europa.eu/document/download/aa535110-c020-4924-8507-5f867adc9972_en
