
Biotechnology
Skin care
peer-reviewed
From Fermented Food to Precision Fermentation for Cosmetic Active Ingredients: An Overview
HARALD VAN DER HOEVEN1, DR. JULE LEXA VÖLZKE2
- Director of Product Design and Development, Chemisches Laboratorium Dr. Kurt Richter GmbH, Germany
- Manager of Product Design and Development, Chemisches Laboratorium Dr. Kurt Richter GmbH, Germany
ABSTRACT: Fermentation has a long history and is now recognized as an important technology for the future. It is a technology that has been part of the cosmetics industry for decades. The use of probiotic bacteria in obtaining cosmetic active ingredients is not new, either. Since the 1980s so-called postbiotics have become established as effective and safe active ingredients. New precision fermentation technologies allow for further improvement of these active ingredients. This implies understanding probiotic bacteria, their cellular constituents and their metabolites. This article aims to supply the reader with a concise overview of history and future of fermentation as a promising and important (bio)technology for cosmetic active ingredients.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
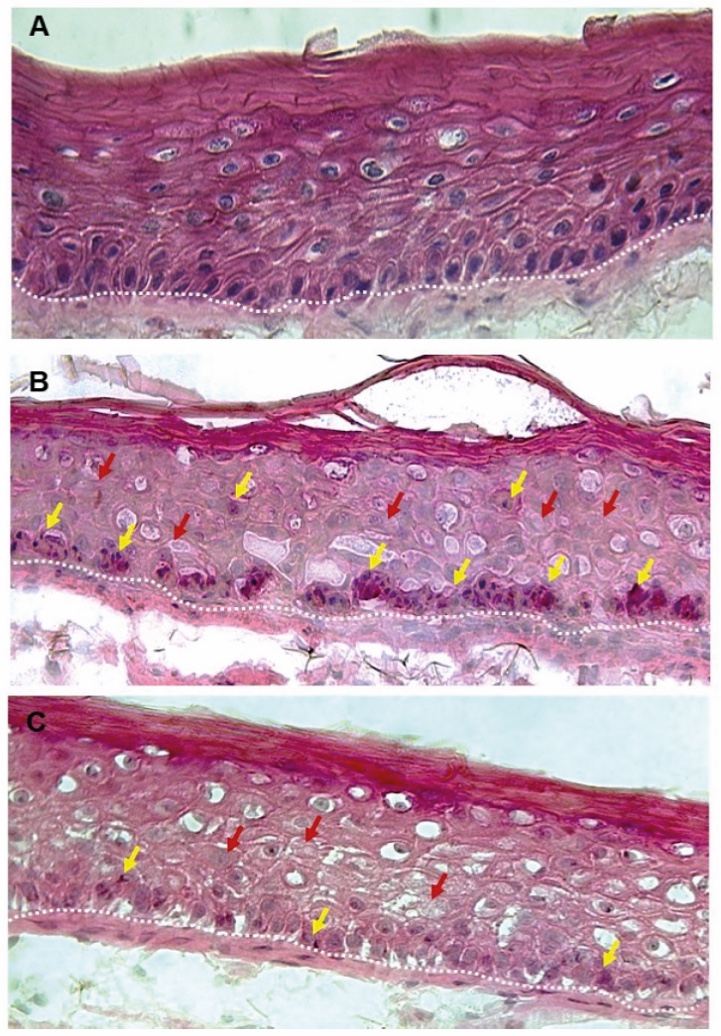
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
History of fermentation
Fermentation is obviously not a new technology. For thousands of years bacteria and yeasts have been used to preserve foodstuffs, making them safe from spoilage (1). This originated in the prehistoric era, when fermentation likely began unintentionally as humans stored food in warm environments in which microbes thrived, transforming the food into a fermented version of its original (2). Later, beverages like beer and wine, and dairy products such as cheese and yoghurt started to emerge, as well as, in Asia, foodstuffs like kimchi, soy sauce and miso.
In the 1850s, Louis Pasteur identified the role of microorganisms in fermentation, paving the way for what we now call modern microbiology and biotechnology (3). Today, fermentation is a (bio)technology which enjoys a great amount of attention from the academic world and the industry alike. It is considered one of the main technologies which pave the road to a more secure, sustainable and healthy future for humankind (4).
Fermentation in the cosmetics industry
Nowadays, fermentation is a well-established technology in the cosmetics industry. Much-loved ingredients, such as hyaluronic acid (5), beta-glucan (6), panthenol (7) and polyglutamic acid (8), can be produced biotechnologically. Functional cosmetic ingredients, such as surfactants and emulsifiers, can also be obtained through fermentation (9), underlining the great potency of this technology for the cosmetics industry.
Currently an important emphasis lies on what many people call “probiotic active ingredients.” These are active ingredients, i.e., ingredients which have advantageous outcomes when applied on skin, scalp or hair. The term “probiotic” is recognizable for the consumer and has clear positive connotations. Building on the trust from the food sector and the associated known health benefits, many consumers perceive “probiotics” as safe and healthy. Taken quite literally, however, these active ingredients are not probiotic. The term implies that these ingredients contain live and viable probiotic cells. Most of them, however, are based on lysates or filtrates containing cellular components of, and/or metabolites produced by, these beneficial microbes. They are properly called “postbiotics” or “paraprobiotics.”
Definitions
At the time of writing, two different definitions of “postbiotics” are circulating. An important source of definitions for pre-, pro- and postbiotics is ISAPP (International Scientific Association for Probiotics and Prebiotics, https://isappscience.org/), which defines postbiotics as follows (10): “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host.” In its publication from 2021, this definition further specified that postbiotic ingredients are derived from microorganisms, deliberately inactivated microbial cells with or without metabolites or cell components. In this publication ISAPP also explained what it does not consider to be postbiotic: viruses and bacteriophages, vaccines, filtrates without cell components, purified microbial components (e.g., proteins, peptides, exopolysaccharides), purified microbial metabolites (e.g., organic acids).
There is also another definition. The ICCR (International Cooperation on Cosmetics Regulation, https://www.iccr-cosmetics.org/) is a voluntary international group of cosmetics regulatory authorities from Brazil, Canada, Chinese Taipei, the European Union, Israel, Japan, Republic of Korea, and the United States that meets on an annual basis to discuss cosmetics safety and regulation.
The ICCR defines postbiotics as “inanimate ingredients of microbial origin added to a cosmetic product with an intended cosmetic benefit. These components can either be cells or cell fractions, a filtrate of a fermentation or a metabolite of a microorganism” (11). ICCR sees “paraprobiotics” as a subgroup of “postbiotics.” It can be argued that its definition of “paraprobiotics” equals the ISAPP’s definition of “postbiotics.” ICCR’s definition of “paraprobiotics”: “Ingredients derived from inactivated probiotic microorganisms added to a cosmetic product with an intended cosmetic benefit. Paraprobiotics can either be inactivated microbial cells or components of cellular structures (e.g., cell walls), with or without metabolites” (11).
History of postbiotics in the cosmetics industry
For the sake of clarity, when we talk about postbiotics, we apply ISAPP’s definition. Postbiotics are part of what can be interpreted as a movement, and not a trend, in the cosmetics industry. Over the last years, many new postbiotic cosmetic active ingredients have been launched onto the market by many different suppliers. Some of these launches have been successful and others not. The use of postbiotic is, however, not new in the cosmetics industry. Already in the 1980s, the first skincare products which contained these ingredients appeared on the market. A number of these products are still available, some of which enjoy great attention by consumers worldwide.
A first scientific publication on the use of postbiotics in topical cosmetic applications appeared back in 1982. Presented at the yearly congress of the German Dermatology Society (Deutsche Dermatologische Gesellschaft), this paper (12) describes the effect of “inactivated cultures of the species Bifidobacterium” (Bifida Ferment Lysate) on UV-induced immunosuppression and DNA damage in the context of this ingredient’s potential skin anti-aging effect. This activity was later reported in another paper from 2008. (13) as well. Interestingly, this ingredient was also the topic of scientific publications related to sensitive skin in 2010 (14) and skin barrier in 2023 (15). During the 40-odd years that postbiotic cosmetic active ingredients have been on the market, an ingredient such as Bifida Ferment Lysate clearly has left its mark on the skincare industry as a well-established ingredient with a proven track record.
The search for cause and effect
As is generally the case with many ingredients on the marketplace, the chemical composition of postbiotic cosmetic active ingredients is, by definition, complex. Understanding their mechanism of action on skin is a critical aspect of ensuring these ingredients’ effectiveness as well as safety. On the other hand, the full elucidation of the interaction between microbial constituents and human cells is not just of interest for the cosmetics industry, but also for the food industry. The food industry has arguably been trying to understand the interplay between probiotic bacteria and human cells for much longer than the cosmetics industry. Up until now, the food industry has not been able to fully answer all its scientific questions. All in all, the bars are set high. Luckily, new scientific publications of high relevance for the cosmetics and food industries appear regularly. Importantly, one of the main messages of these papers is that probiotic bacterial cells do not have to be alive to exert most of their benefits for human cells and organs (16, 17). This does make sense, as interaction between cells, one of them dead (and lyzed) or both of them alive, is by definition of a biochemical and cell biological nature.
Molecules originating from one cell type can have an influence on the other cell type and vice versa (18). It is of great interest to understand which molecules that originate from postbiotics have what cell biological influence on human skin cells and skin as a whole. This knowledge is not just of academic interest, but can also help modify and improve biotechnological fermentation processes toward new and further improved postbiotic cosmetic active ingredients. “Wild fermentation” can then become “precision fermentation.”
Probiotic metabolites
Most probiotic bacteria produce large amounts of lactic acid. They do so to gain and maintain a beneficial environment in which they can thrive. The topical application of lactic acid has multiple benefits, making lactic acid an interesting metabolite of probiotics and constituent of postbiotics (19). Probiotic bacteria produce many other metabolites with biological activity. Other acids like, for instance, short chain fatty acids (SCFA’s) are produced by probiotic bacteria and can be constituents of postbiotic cosmetic active ingredients (20). An important disadvantage of the SCFA’s butyric acid, propionic acid, acetic acid and isovaleric is their characteristic odor, but they can potently interact with human cells’ G-protein coupled receptors (21) and peroxisome proliferator-activated receptor-γ (PPARγ, 22) and even with olfactory receptors (23). Through these interactions they activate cellular MAPK (24) and JAK pathways (25), leading to cosmetic benefits. They themselves can have anti-oxidant properties as well (26).
Derivatives of lactic acid, such as ethyl lactate and propyl lactate, are other biologically active molecules which can be produced by probiotic bacteria and be part of postbiotics. Like SCFA’s, they too can interact with G-protein coupled receptors (27). Another derivative of lactic acid, phenyllactic acid, can be produced when phenylalanine is made available to certain types of probiotic bacteria during the fermentation process (28). This molecule is of interest as it has some potent anti-inflammatory (29) and anti-oxidant properties (30). Phenyllactic acid is also described as a potent tyrosinase inhibitor (31), making it an interesting candidate for skin lightening active ingredients. When 4-hydroxyphenylacetic acid is made available to certain types of probiotic bacteria, it can be converted into ferulic acid (32). As a phenolic acid, ferulic acid is a somewhat sensitive molecule, but can act as a strong anti-oxidant (33).
An important group of biologically active metabolites in postbiotics are the indoles (34). Examples of interesting molecules are indole-3-lactic acid, indole-3-carboxaldehyde and 3-hydroxyindole. Indoles are derivatives of tryptophan, produced to the probiotic fermentation process with a multitude of potential benefits for human skin (35). A molecule such as indole-3-lactic acid alone can interact with human skin cells in many different ways. Like ethyl lactate, indole-3-lactic acid interacts with aryl hydrocarbon receptors (36, 37).
Yet another interesting group of probiotic metabolites and postbiotic constituents are the lactones (38). Lactones are cyclic esters formed by the intramolecular condensation of a hydroxyl group and a carboxylic acid group within the same molecule which can have specific antimicrobial activity (39). They are characterized by their ring structures, which can vary in size. Examples of lactones which can be metabolites obtained from fermentation of probiotic bacteria are 3-hydroxybutyryl-L-homoserine lactone and hexanoyl-L-homoserine lactone. A large group of amino acids, mainly glycine and its derivatives, such as threonine, alanine and serine, can also be a product of fermentation of probiotic bacteria (40). The diamine putrescine (41) and its polyamine derivatives spermidine and spermine (42) have also been recognized as biologically active metabolites in postbiotics. Other groups of metabolites with well-known beneficial effects on human cells and organs have been discovered in postbiotics as well. Vitamins are an important group of singular molecules which can be part of the final postbiotic ingredient after fermentation of certain probiotic microbes, for instance (43, 44).
Probiotic cellular constituents
Adding to the endless complexity and possibilities, not only single molecules can have an influence on human cells, constituents of the bacterial cell itself can have potent effects, too. Toll-like receptors (TLRs) are so-called pattern recognition receptors and, as such, are a crucial component of the innate immune system in human skin (45). Peptidoglycans are an important constituent of the probiotic bacterial cell (46). Among other effects, they provide structural support for the maintenance of cell shape and osmotic protection, but they also interact with TLR2 on skin cells (47). Another example is bacterial DNA, which can interact with TLR9 (48). There are, however, many further examples of probiotic cellular constituents which can interact with pattern recognition receptors on skin cells.
Overall, much is known about probiotic bacteria, their metabolites and cellular components, as well as the constituents of postbiotics. Cosmetic postbiotic active ingredients are, and have been for a long time, established in the marketplace. They have shown to work and to be safe to use. Together with some peptides and vitamin derivatives, such as niacinamide and retinol, postbiotics can arguably be interpreted as one of the few categories of cosmetic active ingredients with a real and proven track record. There is, however, still much to discover.
Conclusion
Without any doubt, the future lies in precision fermentation. Imagine postbiotics as an orchestra of metabolites and cellular constituents which, together, generate the “symphony,” i.e., the biological activity of the postbiotic active ingredient. Understanding precision fermentation, i.e., understanding how to “make sure that the orchestra does justice to the piece to be played,” represents the search for the Holy Grail. This requires knowledge, experience and scientific creative thinking while never losing sight of the safety of the end product. With bifida ferment lysate in 1982, postbiotic cosmetic active ingredients had a flying start, and rightly so. Science has commenced and consumers have evolved into well-informed and critical users of cosmetic products. These changes also make up postbiotics, from the past to the present, with great potential for the future.
Conclusion
The future of cosmetics lies in the continued evolution of holistic approaches which represents a transformative shift in the industry, merging scientific advancements, natural ingredients, and wellness principles. By understanding and embracing the interconnectedness of these elements, the cosmetics industry can cultivate products that not only enhance external beauty but also contribute to the overall well-being of individuals and the planet.
The interplay between beauty from within and topical cosmetics is the key for future products. The integration of biotechnology and green chemistry is revolutionizing cosmetic formulations, offering sustainable and biocompatible alternatives.
Developers can implement blockchain to trace the journey of ingredients from source to product. Nevertheless, the efficacy of the natural products should be scientifically proven. Marketers can communicate transparency as a brand value, and parallelly educate consumers by highlighting how specific ingredients contribute to radiant and healthy skin.
By embracing the synergy between these approaches and leveraging scientific advancements, the cosmetics industry can provide consumers with comprehensive beauty solutions that cater to both internal and external dimensions of beauty.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
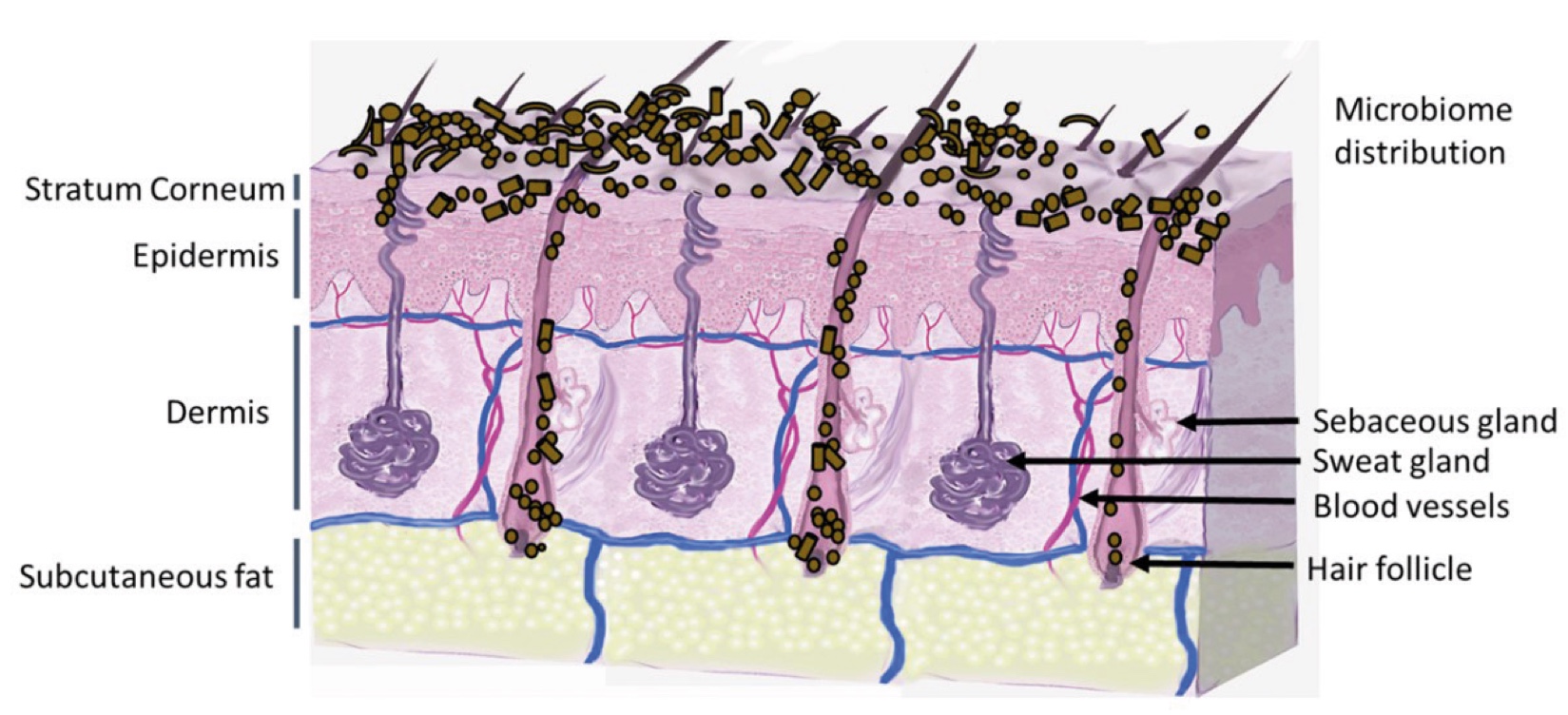
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Zapaśnik A et al. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods. 2022 Apr 28;11(9):1283. doi: 10.3390/foods11091283. PMID: 35564005; PMCID: PMC9099756.
- Liu L, et al. The origins of specialized pottery and diverse alcohol fermentation techniques in Early Neolithic China. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12767-12774. doi: 10.1073/pnas.1902668116. Epub 2019 Jun 3. PMID: 31160461; PMCID: PMC6600912.
- Toledo-Pereyra LH. Louis Pasteur surgical revolution. J Invest Surg. 2009 Mar-Apr;22(2):82-7. doi: 10.1080/08941930902794729. PMID: 19283609.
- Teng TS et al. Fermentation for future food systems: Precision fermentation can complement the scope and applications of traditional fermentation. EMBO Rep. 2021 May 5;22(5):e52680. doi: 10.15252/embr.202152680. Epub 2021 Apr 27. PMID: 33908143; PMCID: PMC8097352.
- Serra M et al. Microbial Hyaluronic Acid Production: A Review. Molecules. 2023 Feb 23;28(5):2084. doi: 10.3390/molecules28052084. PMID: 36903332; PMCID: PMC10004376.
- Singla A et al. Beta-Glucan as a Soluble Dietary Fiber Source: Origins, Biosynthesis, Extraction, Purification, Structural Characteristics, Bioavailability, Biofunctional Attributes, Industrial Utilization, and Global Trade. Nutrients. 2024 Mar 21;16(6):900. doi: 10.3390/nu16060900. PMID: 38542811; PMCID: PMC10975496.
- https://patents.google.com/patent/WO2007131750A1/en
- Gou Y et al. Investigation of γ-polyglutamic acid production via asynchronous saccharification and fermentation of raw corn starch. World J Microbiol Biotechnol. 2024 Oct 3;40(11):338. doi: 10.1007/s11274-024-04141-5. PMID: 39358620.
- Nasser M et al. Advances in the production of biosurfactants as green ingredients in home and personal care products. Front Chem. 2024 Mar 26;12:1382547. doi: 10.3389/fchem.2024.1382547. PMID: 38595700; PMCID: PMC11002128.
- Salminen S et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021 Sep;18(9):649-667. doi: 10.1038/s41575-021-00440-6. Epub 2021 May 4. An interesting infographic helps to interpret this definition: https://isappscience.org/postbiotics-inforgraphic/
- https://www.iccr-cosmetics.org//downloads/topics/2022-06%20-%20microbiome%20and%20cosmetics-working%20definitions%20%20microbiological%20assessment%20considerations.pdf
- Born W et al. Stimulation der Repair-Synthese Gegen bleibende DNA-Schäden. Der Hautarzt, 1982: 102-105.
- Lucas CR et al. Immune protective effect of a moisturizer with DNA repair ingredients. J Cosmet Dermatol. 2008 Jun;7(2):132-5. doi: 10.1111/j.1473-2165.2008.00376.x. PMID: 18482017
- Guéniche A et al. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp Dermatol. 2010 Aug;19(8):e1-8. doi: 10.1111/j.1600-0625.2009.00932.x. PMID: 19624730.
- Wang R et al. The pivotal role of Bifida Ferment Lysate on reinforcing the skin barrier function and maintaining homeostasis of skin defenses in vitro. J Cosmet Dermatol. 2023 Dec;22(12):3427-3435. doi: 10.1111/jocd.15831. Epub 2023 May 23. PMID: 37218728.
- Vinderola G et al. Postbiotics: The concept and their use in healthy populations. Front Nutr. 2022 Dec 9;9:1002213. doi: 10.3389/fnut.2022.1002213. PMID: 36570166; PMCID: PMC9780264.
- Tsilingiri K et al. Postbiotics: what else? Benef Microbes. 2013 Mar 1;4(1):101-7. doi: 10.3920/BM2012.0046. PMID: 23271068.
- N'Diaye A et al. Substance P and Calcitonin Gene-Related Peptide: Key Regulators of Cutaneous Microbiota Homeostasis. Front Endocrinol (Lausanne). 2017 Jan 30;8:15. doi: 10.3389/fendo.2017.00015. PMID: 28194136; PMCID: PMC5277020.
- Salgaonkar N et al. Glycerol fermentation by skin bacteria generates lactic acid and upregulates the expression levels of genes associated with the skin barrier function. Exp Dermatol. 2022 Sep;31(9):1364-1372. doi: 10.1111/exd.14604. Epub 2022 May 25. PMID: 35535416.
- Vinolo MA et al. Regulation of inflammation by short chain fatty acids. Nutrients. 2011 Oct;3(10):858-76. doi: 10.3390/nu3100858. Epub 2011 Oct 14. PMID: 22254083; PMCID: PMC3257741.
- Kimura I et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011 May 10;108(19):8030-5. doi: 10.1073/pnas.1016088108. Epub 2011 Apr 25. PMID: 21518883; PMCID: PMC3093469.
- Hasan AU et al. Interactions between Host PPARs and Gut Microbiota in Health and Disease. Int J Mol Sci. 2019 Jan 17;20(2):387. doi: 10.3390/ijms20020387. PMID: 30658440; PMCID: PMC6359605.
- Depetris-Chauvin A et al. Olfactory detection of a bacterial short-chain fatty acid acts as an orexigenic signal in Drosophila melanogaster larvae. Sci Rep. 2017 Oct 27;7(1):14230. doi: 10.1038/s41598-017-14589-1. PMID: 29079812; PMCID: PMC5660182.
- Matsushita M et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Prostate Cancer Growth via IGF1 Signaling. Cancer Res. 2021 Aug 1;81(15):4014-4026. doi: 10.1158/0008-5472.CAN-20-4090. Epub 2021 May 26. PMID: 34039634.
- Du Y et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int J Mol Sci. 2024 Jul 5;25(13):7379. doi: 10.3390/ijms25137379. PMID: 39000498; PMCID: PMC11242198.
- González-Bosch C et al. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021 Nov;47:102165. doi: 10.1016/j.redox.2021.102165. Epub 2021 Oct 14. PMID: 34662811; PMCID: PMC8577496.
- Peters A et al. Metabolites of lactic acid bacteria present in fermented foods are highly potent agonists of human hydroxycarboxylic acid receptor 3. PLoS Genet. 2019 May 23;15(5):e1008145. doi: 10.1371/journal.pgen.1008145. Erratum in: PLoS Genet. 2019 Jul 19;15(7):e1008283. doi: 10.1371/journal.pgen.1008283. PMID: 31120900; PMCID: PMC6532841.
- Lunavath R et al. Antimycotic effect of 3-phenyllactic acid produced by probiotic bacterial isolates against Covid-19 associated mucormycosis causing fungi. PLoS One. 2023 Mar 30;18(3):e0279118. doi: 10.1371/journal.pone.0279118. PMID: 36996100; PMCID: PMC10062613.
- Lee M et al. Metabolites of Kimchi Lactic Acid Bacteria, Indole-3-Lactic Acid, Phenyllactic Acid, and Leucic Acid, Inhibit Obesity-Related Inflammation in Human Mesenchymal Stem Cells. J Microbiol Biotechnol. 2024 Feb 28;34(2):306-313. doi: 10.4014/jmb.2308.08015. Epub 2023 Oct 31. PMID: 37940180; PMCID: PMC10940772.
- Beloborodova N et al. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J Biomed Sci. 2012 Oct 12;19(1):89. doi: 10.1186/1423-0127-19-89. PMID: 23061754; PMCID: PMC3503878.
- Shin M et al. Investigation of phenyllactic acid as a potent tyrosinase inhibitor produced by probiotics. Curr Res Food Sci. 2022 Dec 9;6:100413. doi: 10.1016/j.crfs.2022.100413. PMID: 36569188; PMCID: PMC9772785.
- Cheng Y et al. A bacterial platform for the production of 4-hydroxyphenylacetic acid and its derivatives. Bioresour Technol. 2025 Feb 16;422:132240. doi: 10.1016/j.biortech.2025.132240. Epub ahead of print. PMID: 39965711.
- Zheng M et al. The Antioxidant Properties, Metabolism, Application and Mechanism of Ferulic Acid in Medicine, Food, Cosmetics, Livestock and Poultry. Antioxidants (Basel). 2024 Jul 16;13(7):853. doi: 10.3390/antiox13070853. PMID: 39061921; PMCID: PMC11273498.
- Ye X et al. Dual Role of Indoles Derived From Intestinal Microbiota on Human Health. Front Immunol. 2022 Jun 17;13:903526. doi: 10.3389/fimmu.2022.903526. PMID: 35784338; PMCID: PMC9248744.
- Pan T et al. Uncovering the specificity and predictability of tryptophan metabolism in lactic acid bacteria with genomics and metabolomics. Front Cell Infect Microbiol. 2023 Mar 13;13:1154346. doi: 10.3389/fcimb.2023.1154346. PMID: 36992687; PMCID: PMC10040830.
- Zhang Z et al. Lactobacillus gasseri LGV03-derived indole-3-lactic acid ameliorates immune response by activating aryl hydrocarbon receptor. Microb Cell Fact. 2025 Jan 30;24(1):34. doi: 10.1186/s12934-025-02662-8. PMID: 39885499; PMCID: PMC11780890.
- Kim K et al. Effects of Indole-3-Lactic Acid, a Metabolite of Tryptophan, on IL-4 and IL-13-Induced Human Skin-Equivalent Atopic Dermatitis Models. Int J Mol Sci. 2022 Nov 4;23(21):13520. doi: 10.3390/ijms232113520. PMID: 36362303; PMCID: PMC9655012.
- Zia H et al. Biotechnological formation of dairy flavor inducing δ-lactones from vegetable oil. Food Chem X. 2022 Jan 19;13:100220. doi: 10.1016/j.fochx.2022.100220. PMID: 35498959; PMCID: PMC9039933.
- Mazur M et al. Antimicrobial Activity of Lactones. Antibiotics (Basel). 2022 Sep 29;11(10):1327. doi: 10.3390/antibiotics11101327. PMID: 36289985; PMCID: PMC9598898.
- Bernardo D et al. Microbiota/host crosstalk biomarkers: regulatory response of human intestinal dendritic cells exposed to Lactobacillus extracellular encrypted peptide. PLoS One. 2012;7(5):e36262. doi: 10.1371/journal.pone.0036262. Epub 2012 May 14. PMID: 22606249; PMCID: PMC3351486.
- Hirano R et al. Putrescine Production by Latilactobacillus curvatus KP 3-4 Isolated from Fermented Foods. Microorganisms. 2022 Mar 24;10(4):697. doi: 10.3390/microorganisms10040697. PMID: 35456748; PMCID: PMC9026525.
- Kitada Y et al. Bioactive polyamine production by a novel hybrid system comprising multiple indigenous gut bacterial strategies. Sci Adv. 2018 Jun 27;4(6):eaat0062. doi: 10.1126/sciadv.aat0062. PMID: 29963630; PMCID: PMC6021145.
- LeBlanc JG et al. B-group vitamin production by lactic acid bacteria--current knowledge and potential applications. J Appl Microbiol. 2011 Dec;111(6):1297-309. doi: 10.1111/j.1365-2672.2011.05157.x. Epub 2011 Oct 10. PMID: 21933312.
- Rossi M et al. Folate production by probiotic bacteria. Nutrients. 2011 Jan;3(1):118-34. doi: 10.3390/nu3010118. Epub 2011 Jan 18. PMID: 22254078; PMCID: PMC3257725.
- Fitzgerald KA et al. Toll-like Receptors and the Control of Immunity. Cell. 2020 Mar 19;180(6):1044-1066. doi: 10.1016/j.cell.2020.02.041. Epub 2020 Mar 11. PMID: 32164908; PMCID: PMC9358771.
- Dziarski R. Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci. 2003 Sep;60(9):1793-804. doi: 10.1007/s00018-003-3019-6. PMID: 14523544; PMCID: PMC11138782.
- Takeda K et al. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res. 2002;8(6):459-63. doi: 10.1179/096805102125001073. PMID: 12697090.
- Cornélie S et al. Direct evidence that toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific cytosine-phosphate-guanine motif recognition. J Biol Chem. 2004 Apr 9;279(15):15124-9. doi: 10.1074/jbc.M313406200. Epub 2004 Jan 20. PMID: 14736866.

