
Regulation
on
Skin care
peer-reviewed
An overview on how the European Green Deal will affect the cosmetic industry and tips to be ready
OLIVIA SANTONI, LAILA MANSHI
Bloom Regulatory Ltd, London, England
Article received on April 3, 2024
ABSTRACT: The European Green Deal (1) is an integral part of the European Commission’s strategy to implement the United Nations 2030 Agenda. It will affect many policy areas, industry sectors, and includes individual initiatives.
In particular, this initiative will transform the way we regulate and assess chemicals within the EU via the Chemicals Strategy for Sustainability (CSS).
Alongside the CSS sits the Circular Economy Action Plan (CEAP) which targets product-design, circular economy processes, sustainable consumption, waste prevention, and resource retention. For cosmetics, this will impact the formulation, the packaging composition and design, and will require further transparency by providing information to consumers to make sustainable choices including rules around green claims. This article will provide an overview on how the European Green Deal will affect the cosmetic industry and tips for businesses to start getting ready.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
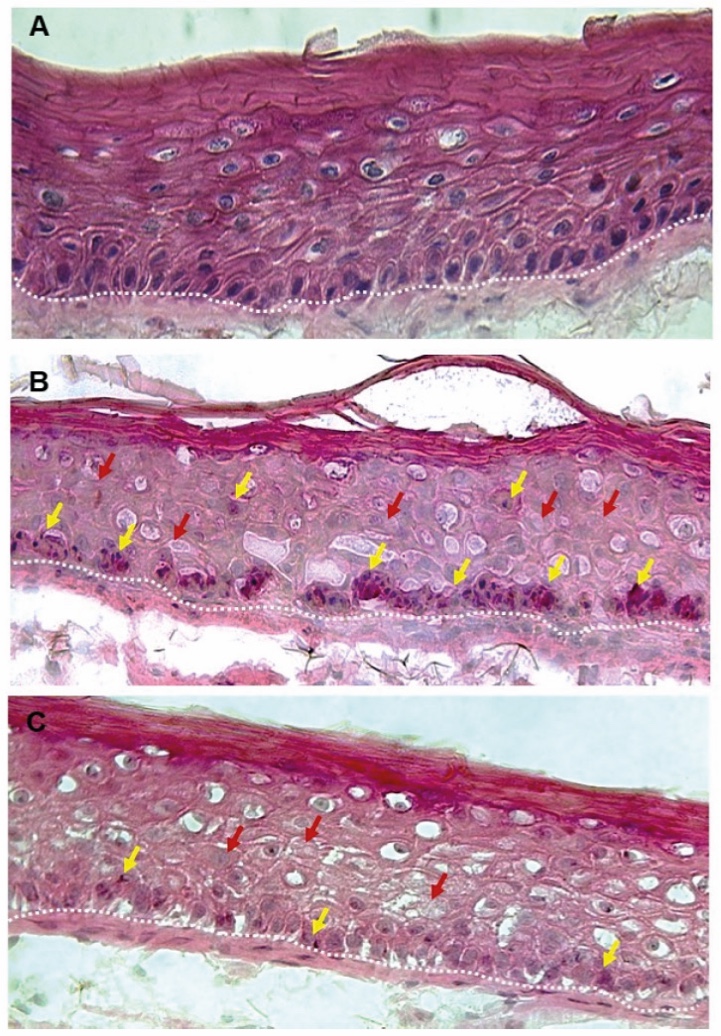
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction
The European Green Deal (1) (EGD) is a new growth strategy that aims to transform the European Union (EU) into a fair and prosperous society, with a modern, resource-efficient and competitive economy where there are no net emissions of greenhouse gases in 2050 and where economic growth is decoupled from resource use.
The zero-pollution vision for 2050 is for air, water and soil pollution to be reduced to levels no longer considered harmful to health and natural ecosystems, that respect the boundaries with which our planet can cope, thereby creating a toxic-free environment.
The EGD announced headline (2) actions on zero pollution:
- Chemicals Strategy for Sustainability (CSS); to better protect citizens and the environment against hazardous chemicals.
- Zero pollution action plan for water, air, and soil; to better prevent, remedy, monitor and report on pollution.
- Revising measures to address pollution from large industrial installations and to ensure they are consistent with climate, energy and circular economy policies.
The action plan aims to strengthen the EU green, digital and economic leadership, whilst creating a healthier, socially fairer Europe and planet. It provides a compass to mainstream pollution prevention in all relevant EU policies, to step up implementation of the relevant EU legislation and to identify possible gaps.

Main contents
An overview of the EGD
The EGD is an integral part of the European Commission's (EC) strategy to implement the United Nations 2030 Agenda and the sustainable development goals. The Green Deal will affect many policy areas, spanning all industry sectors, and includes many individual initiatives to put the EU at the forefront of sustainability and green matters. Formula and ingredient sourcing will be severely affected by the CSS. In particular it will require the revision of legislation including; Registration, Evaluation, Authorisation and Restriction of Chemicals (REACh), Classification, Labelling and Packaging (CLP) and the Cosmetics Product Regulation (CPR) (as well as other sector-specific legislation).
css
Traditionally, chemical safety in the EU has been managed through two primary risk management approaches: Specific Risk Assessment and Generic Risk Approach (GRA). While both methods aim to ensure a high level of protection for human health and the environment, they differ in their implementation.
- ‘Specific risk assessments’ consider the hazard, the use of the substances and related specific exposure scenarios for humans and the environment (risk), and bespoke / specific risk management measures are triggered based on their outcomes.
- ‘Generic approach to risk management’ is an automatic trigger of pre-determined risk management measures (e.g. packaging requirements, restrictions, bans, etc.) based on the hazardous properties of the chemical and generic considerations of their exposure.
The CSS seeks to harmonise chemical management practices across sectors, transitioning from specific risk assessments to a GRA approach as well as utilising grouping methodologies. This extension of the use of the GRA will have a huge impact on formula management and innovation as groups of hazardous substances will end up being automatically regulated based on their hazard.
Circular Economy Action Plan (CEAP)
In March 2020, the EC adopted the new CEAP. It is one of the main building blocks of the European Green Deal. The EU’s transition to a circular economy will reduce pressure on natural resources while fostering sustainable growth and jobs. It is also a prerequisite to achieve the EU’s 2050 climate neutrality target and mitigating biodiversity loss.
The new action plan announces initiatives along the entire life cycle of products. It focuses on product design, promotes circular economy practices, encourages sustainable consumption, and aims to minimise waste generation while maximising resource retention within the EU economy.
Impact of the Chemicals Strategy for Sustainability (CSS) on cosmetics businesses
The CSS is going to totally change the way we manage chemicals in Europe by implementing a harmonisation across all sectors, moving away from specific risk assessments to a GRA. This extension of the use of the GRA will have a huge impact on formula management and innovation as groups of hazardous substances will end up being automatically regulated based on their hazard.
For cosmetics we are expecting the GRA to go beyond Carcinogenic, Mutagenic and Reprotoxic Chemicals (CMRs) and to ban or restrict the use of further hazard classes, while allowing limited exemptions (notably Essentiality and Mixture Assessment Factors (MAFs)).
Cosmetics businesses have to start monitoring CLP harmonised classifications and self-classifications to anticipate any requirement to reformulate in the future. When selecting new ingredients for use, it will also be advisable to assess CLP classifications.
If exemptions are permitted for cosmetics, it will be important to design products with only 'essential' ingredients (in case of classification) and to avoid those with borderline Margins of Safety (MoS), as these are likely to be most at risk from application of a MAF.
Whilst the extension of GRA is something we anticipate under the revision of the chemicals strategy in the EU, some GRA issues are already progressing through the regulatory channels. Namely, the addition of Endocrine Disruptors to the CPR, widening of the scope for nanomaterials and restriction on microplastics and PFAs use.
Also, it is important to note that for efficiency purposes, the grouping of substances (3) has been identified by the EU Commission as a useful tool in chemical hazard assessment as it allows to predict properties of substances without having to test them all for each endpoint (‘read-across’).
This initiative is changing the way we have been managing cosmetics ingredients as potential bans of ingredients may, in the future, be based on chemical grouping assessment. Because the GRA may address a group of substances based on their hazards or other characteristics, ingredient defence will be more challenging for the industry.
The grouping of substances could mean that a whole group of ‘similar’ substances may be classified based on the presence of one ‘bad apple’ in the group. Some groupings will result in scientific false positive conclusions (grouping method is still under discussion). Therefore, a batch of substances will be banned or restricted automatically, and the industry will have a limited time to act to propose an exemption. Also, even in case of an exemption being granted from a legal perspective the use of the ingredient may not be suitable from a consumer/retailer expectation point of view.

Impact of the Circular Economy Action Plan (CEAP) on cosmetics businesses
The CEAP is an integral part of the EGD to reduce pressure on natural resources and create sustainable growth and achieve the EU’s 2050 climate neutrality target and to halt biodiversity loss. These big ambitions will be transposed via several new legislations (or amendments to current legislations). In particular relevance for our sector (among other laws) we can highlight today are the Green Claims Directive (4), the Packaging and Packaging Waste Regulation (5) (PPWR) and the Regulation on Eco-design for Sustainable Products (6).
Green Claims Directive:
This new directive is under the legislative process, and we are awaiting the final approval from the EU Council, after which it will be published in the Official Journal of the EU.
This Directive will impact all sectors significantly, in particular, in relation to the burden of proof relating to green claims to protect consumers against greenwashing. Companies will need to audit all claims currently made and determine whether the claim is compliant to the new rules and has sufficient and appropriate substantiation available. In particular we are expecting the ban on the use of unsubstantiated generic environmental claims such as ‘environmentally friendly’, ‘natural’, ‘biodegradable’ and ‘eco’.
Regulation on Eco-design for Sustainable Products
The Regulation on Eco-design for Sustainable Products sets new requirements to make products more durable, reliable, reusable, upgradable, reparable, easier to maintain, refurbish and recycle, and energy and resource efficient.
In addition, product-specific information requirements will ensure consumers know the environmental impacts of their purchases. All regulated products will have Digital Product
Passports (DPP). This will make it easier to repair or recycle products and facilitate tracking substances of concern along the supply chain. Labelling may be introduced as well. The proposal also contains measures to end the destruction of unsold consumers.
PPWR
As part of the Green Deal, the PPWR is being revised and is expected to enhance the previous provisions by revising requirements as well as being turned into an EU Regulation. Including:
- to improve packaging design to promote reuse and recycling
- for specific recycled content in packaging
- obligations relating to excessive packaging
- obligations relating to packaging waste
- harmonisation of sorting instructions.
Choosing the right packaging and holding the right information and minimisation dossier will become crucial in the future. Companies will not only have to check the compliance of their formula but also to ensure their packaging is compliant with this Regulation. Specific criteria for selecting packaging will have to be introduced internally as well as making sure that the packaging supplier can provide technical information on their product to the supply chain. In particular, cosmetics businesses will have to know the composition, the recycled content and the specifications of the packaging they will use. Choosing the right product and the right partner will be important.
How to start preparing for this new complex regulatory and policy landscape
As businesses brace themselves for the complexities of shifting regulatory and policy frameworks, proactive steps can be taken to ensure that they are ready.
Ingredient selection
Monitor and understand hazard categories: Begin by comprehensively assessing and understanding your ingredient portfolio’s hazard categories and implementing a hazard check for new substances discussed at R&D / innovation level.
Check existing classifications: Evaluate existing classifications under the Classification, Labelling, and Packaging Regulation (CLP), along with any self-classifications provided by suppliers. Additionally, be aware of classifications coming from other suppliers that could impact the CLP harmonised classifications in the future. Monitor the process of new harmonised CLP classifications and any cosmetic-specific review by the SCCS.
Essentiality: Consider whether the material is essential in the product. Does it have a function?
MAF: Understand the calculated Margin of Safety relating to the ingredients in your formulas.
Consumer Perception: Not all classifications are considered equal by consumers. Therefore, some pending classifications may be deemed a greater PR risk than others when permission to continue use may be possible under the Cosmetics Regulation. If using a ‘blacklist’ of ingredients/categories of ingredients to avoid, it is advisable to include a wide range of internal personnel to ensure that emotive decisions do not unduly create restrictions on innovation and product performance. Honesty and truthfulness should remain fundamental to any consumer communications.
Future sustainability: When choosing more ‘friendly’ alternatives it is important to avoid the risk of ‘jumping out of the frying pan and into the fire’. In recent years we have already seen the results of industry choices and how they can create new problems when seeking to remove the original issue e.g. the move from parabens to Methylisothiazolinone (MIT) resulted in an increase of consumer sensitivity to MIT and subsequent regulatory restrictions.
Claims
Audit your green claims: Audit your packaging and marketing communications and establish what green claims you are making.
Avoid vague and broad green claims: Generic environmental claims such as ‘environmentally friendly’, ‘natural’, ‘biodegradable’ and ‘eco’ could mislead consumers as to the environmental impact of your product and are likely to be targeted by regulatory authorities. In particular, avoid any claims that imply a positive impact on the environment as generally production of any kind is never good for the environment!
Substantiation: Ensure that you hold robust substantiation for any green claims made that will hold up under regulatory scrutiny. Expect to be challenged for any bold green claims and ensure you have the right evidence to support!
Consider the full life cycle of the product: Consider environmental impacts at every stage, from sourcing raw materials to manufacturing, use, and disposal. The authorities will expect claims to relate to the full life cycle of the product and so it is important to transparently communicate efforts to minimise environmental footprint across the entire lifecycle.
Consumer awareness and transparency: Consumers are increasingly aware of ‘greenwashing’ and want honest and open communication with the brands they purchase from. Provide clear and accessible information to empower consumers to make informed choices about the environmental impact of products. It is equally important to not omit any information that will help guide consumers to their purchase decision.
Packaging
Evaluate your current packaging portfolio: To prepare for the PPWR, establish your full packaging portfolio including each product's packaging components, what material each component is made of and whether they are recyclable or made from recycled content. Find out from your packaging suppliers whether any components contain hazardous substances e.g. endocrine disruptors, SVHCs or heavy metals.
EPR scheme: Determine whether your business is part of an Extended Producer Responsibility (EPR) scheme, which places responsibility on producers to manage the environmental impact of their packaging waste. Engage actively in EPR initiatives to optimise packaging design, enhance recycling infrastructure, and promote circularity within the packaging lifecycle.
Single-Use products: Assess whether your product range includes single-use hotel miniature packaging, particularly for hygiene and toiletry products. Be mindful of regulatory thresholds under the PPWD, such as volumes of less than 50 ml for liquid products or less than 100 g for non-liquid products and explore opportunities to minimise single-use packaging through innovative design or alternative packaging formats.
By proactively addressing these considerations, businesses can navigate the evolving regulatory and policy landscape with greater confidence and resilience.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
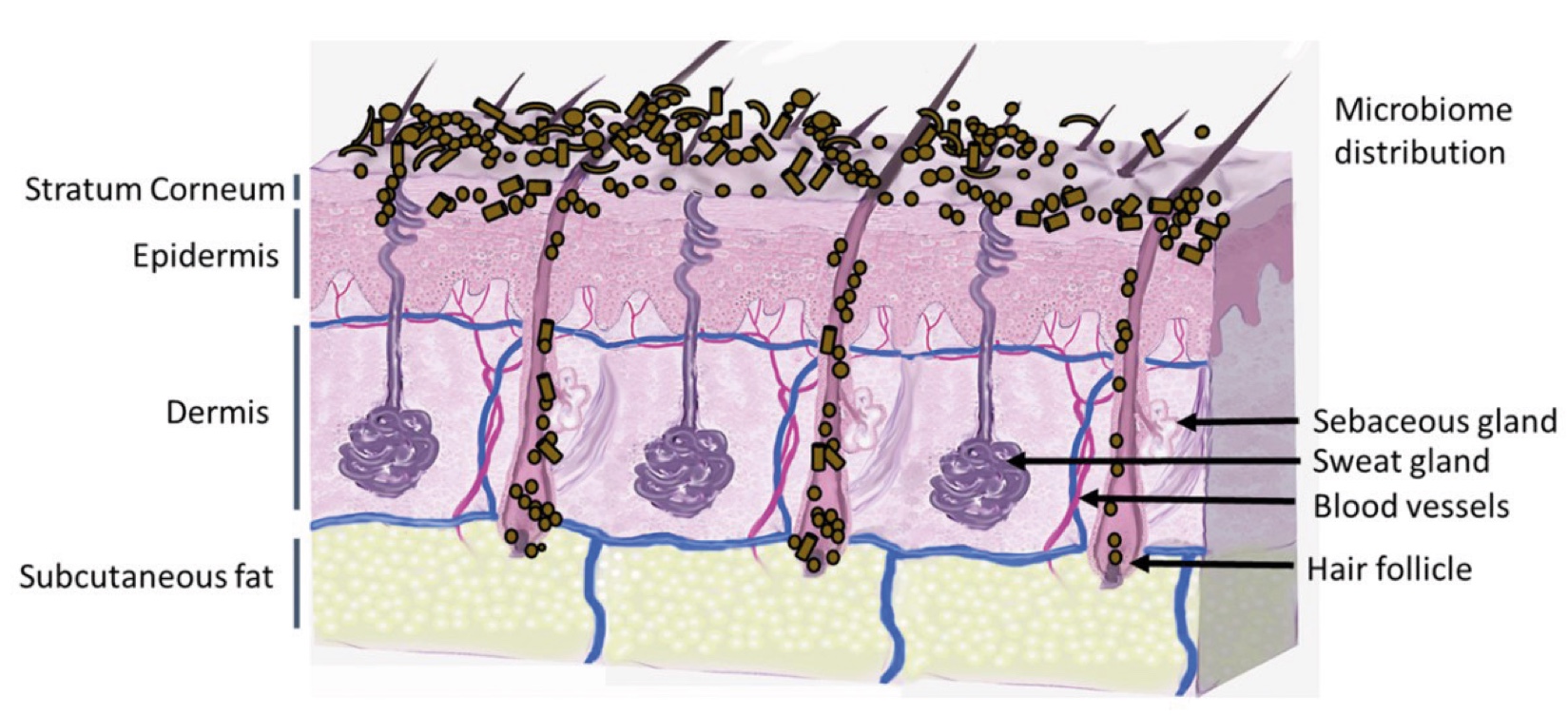
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- The European Green Deal - European Commission (europa.eu)
- Zero Pollution Action Plan - European Commision (europa.eu)
- Working with Groups - ECHA (europa.eu)
- Green claims - European Commission (europa.eu)
- Packaging waste - European Commission (europa.eu)
- Ecodesign for Sustainable Products Regulation - European Commission (europa.eu)

