
Regulation
on
Skin care
peer-reviewed
Where are we in the development of the new European Cosmetics Regulation?
FRÉDÉRIC LEBREUX
BIORIUS, Wavre, Belgium
Article received on March 26th
ABSTRACT: In the realm of cosmetics legislations, the European Cosmetics Regulation EC N°1223/2009 has long been a cornerstone of consumer safety and ethical standards. This article examines the imperative for its recast, exploring anticipated changes such as expanded risk assessment methodologies and integration of new nanomaterial definitions. Delving into stakeholder perspectives and regulatory timelines, it navigates the complexities of this evolving landscape, emphasizing the commitment to transparency, innovation, and market integrity. As Europe prepares to usher in a new era of cosmetics regulation, stakeholders are poised at a pivotal juncture, balancing challenges and opportunities to ensure consumer protection and industry sustainability.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction: the role and priorities of the current European Cosmetics Regulation EC N°1223/2009
In today's globalized world, the European Cosmetics Regulation EC N°1223/2009 [1] is pivotal, ensuring transparency, safety, and ethics in the cosmetics industry within the European Union. It prioritizes consumer health through stringent safety assessments, allergen checks, and ingredient evaluations, instilling confidence in daily product use.
Mandated clear labeling empowers consumers with information, promoting transparency and accountability. The regulation aligns with European values by opposing animal testing, regulating nanomaterials, and fostering ethical practices and environmental sustainability.
By establishing harmonized standards, the regulation also facilitates cross-border trade, fostering fair competition and economic growth while safeguarding consumer and business interests.
And yet, this legal instrument that embodies the EU's dedication to consumer protection, ethics, and market integrity, enriching lives across Europe will soon get revised.
Towards a paradigm shift: Why to recast the European Cosmetics Regulation?
As cosmetics evolve alongside technology and consumer expectations, the European Commission seeks to revamp the regulatory framework. The recast of the European Cosmetics Regulation EC N°1223/2009 aims to address challenges, enhance consumer protection, and promote innovation amidst the European Green Deal and Chemical Strategy for Sustainability.
Updating the regulation becomes crucial as the society changes, the state of our knowledge evolves and new political directions are taken – starting with a strong ambition to better protect the environment. The recast aims to modernize the framework, adapting to scientific progress and consumer preferences to ensure effectiveness in protecting health, rights, and values.
While the Commission’s regulatory proposal has yet to come, recent communications show us the direction, which seems to align well with the above target. With this article, the current state of play is reported and the speculative exercise to define timelines is conducted.
What is new in the next European Cosmetics Regulation?
Right now, it is difficult to provide certainty on what the next European Cosmetics Regulation will contain since the regulatory proposal has not yet been issued by the Commission. However, communications have been made regularly by Hans Ingels, Head of Unit F2 ( Bioeconomy, Chemicals & Cosmetics) at DG GROW in a very transparent way. At this stage, we expect a targeted revision of the regulation although other scenarios exists as it is explained a bit farther.

Expansion of the Generic Risk Assessment Approach for Highly Hazardous Substances
One of the pivotal anticipated changes pertains to the regulatory framework governing the most hazardous substances in cosmetics. Traditionally, the evaluation of ingredients in these products adheres to the fundamental principles of risk assessment: assessing the inherent hazard of an ingredient – akin to gauging a lion's capacity to cause harm – against consumer exposure levels. This methodology allows for the safe use of ingredients with toxicological properties in cosmetic formulations, provided consumer exposure remains sufficiently low. However, the precautionary principle, enshrined in Article 191 of the Treaty on the Functioning of the EU, mandates proactive risk management measures to safeguard public health in instances where scientific evidence on ingredient toxicity is limited. This principle is notably applied to Carcinogenic, Mutagenic, and Reprotoxic substances (CMRs) in the current regulatory framework.
Under the current regulations, the official classification of an ingredient as a CMR, as per the CLP Regulation EC No. 1272/2008, typically results in its outright prohibition in cosmetics or imposes stringent usage restrictions.
The forthcoming cosmetics regulation aims to broaden this existing linkage with the CLP Regulation to encompass additional classifications. As of the time of writing, there have been discussions surrounding the official classification of certain ingredients as Endocrine Disruptors (for human health), category 1.
Should ingredients receive such a classification, they would face automatic prohibition unless the industry advocates for their continued use. The criteria for exemption from generic risk assessment are delineated in Article 15 of the existing regulation, and the Commission has pledged to clarify the exemption process to afford stakeholders greater certainty.
Furthermore, the classification of an ingredient as a Respiratory Sensitizer (category 1) or as posing Specific Target Organ Toxicity (STOT) (categories 1, 1A, and 1B) is considered a matter of grave concern. At this stage, the Commission proposes that such classifications would trigger deliberations within the European Commission's working groups and at the COSCOM level. Subsequently, the SCCS may be tasked with assessing these ingredients for regulatory action. While progress has been made in broadening the scope of the Generic Risk Assessment approach over the past year, it remains a source of apprehension, potentially endangering numerous cosmetic ingredients currently in use.
Mixture Assessment Factors (MAF)
In line with the Chemical Strategy for Sustainability, the European Commission aims to institute legal provisions addressing the issue of "combined exposure to chemicals," referring to exposure to inadvertent mixtures of various chemicals in the environment. A proposed solution to mitigate risks associated with such exposures is the introduction of a "Mixture Assessment Factor" (MAF). The MAF would be applied to the existing Margin of Safety (MoS) of individual ingredients to ensure that these unintended mixtures maintain safety levels for consumers.
While discussions surrounding MAF have progressed significantly in other regulatory frameworks, the method by which MAF could be integrated into the European Cosmetics Regulation remains unclear. Presently, the most viable approach appears to involve tasking the Scientific Committee on Consumer Safety with developing and proposing best practices for MAF integration within its Notes of Guidance, rather than enshrining it directly into the regulatory text.
This approach allows for greater flexibility in adapting to evolving scientific understanding and industry practices, ensuring that consumer safety remains paramount while facilitating regulatory compliance within the cosmetics sector.
Transfer of responsibility to the European Chemical Agency
The European Cosmetics Regulation currently designates the Scientific Committee on Consumer Safety (SCCS) as the authority responsible for assessing the safety of specific cosmetic ingredients. Their evaluations serve as the foundation for risk management measures implemented by the European Commission through amendments to the regulation's annexes. Comprising primarily of esteemed academics specializing in various facets of regulatory toxicology, the SCCS has amassed significant expertise in the field of cosmetics safety over the years.
Hence, the proposal put forth by the European Commission to transfer this safety assessment mandate to the European Chemicals Agency (ECHA) elicited concern from both industry stakeholders and non-governmental organizations. The ECHA Risk Assessment Committee boasts a stellar reputation and possesses extensive proficiency in environmental toxicology, particularly in the evaluation of endocrine disruptors. Consequently, the European Commission perceived an opportunity to streamline efforts and avoid redundancy (one substance = one assessment) by entrusting the evaluation of cosmetic ingredients to this established entity.
Upon closer examination, the European Commission eventually plans to relocate the SCCS to the ECHA, albeit maintaining its status as an independent committee. This transition reflects a strategic move to leverage the specialized expertise of the ECHA while preserving the integrity and autonomy of the safety assessment process for cosmetic ingredients.
Integration of the new nanomaterials definition
Although a new regulation isn't explicitly required for this adaptation, the European Commission intends to capitalize on this targeted revision to harmonize the definition of nanomaterials with the Recommendation of June 10, 2022 [2].
The implementation of this new definition poses a significant challenge for the cosmetics industry, as ingredients previously not classified as nanomaterials will now fall under this designation. Unlike the previous nanomaterials definition, the new one does not consider solubility, which previously excluded certain cosmetic ingredients. Additionally, the revised definition encompasses particles that are strongly bound or fused to form aggregates, even if the dimensions of such aggregates exceed 100nm. Furthermore, the definition now relies on a specific methodology, namely the number-based size distribution, known for yielding conservative results.
Given the stringent regulations governing these ingredients in the European Cosmetics Regulations and the imperative to have many ingredients reviewed by the SCCS, the industry has proactively engaged in discussions with the European Commission to mitigate potential business uncertainties. The objective is to prevent ingredients from suddenly becoming unlawful, despite being deemed safe the day before. Encouragingly, current developments suggest that the regulation of these new nanomaterials, estimated at 80 to 120 ingredients, will be phased in gradually, akin to the approach taken with hair dyes regulation.
Transition to voluntary digital labeling
Following extensive deliberations spanning several years, the introduction of new fragrance allergens [3] mandates their labeling on product packaging, presenting a considerable challenge for the industry due to the limited space available on labels.
Amidst calls from industry stakeholders and likely many consumers for the digitalization of labeling requirements, concerns have been raised by the European Commission and certain consumer associations regarding the potential exclusion of individuals less adept with modern technologies.
The forthcoming targeted revisions of the regulation offer an opportune moment to introduce voluntary digital labeling. Both regulatory authorities and industry players have expressed openness to this approach. Hans Ingels, the relevant head of unit at the Commission, recently [4] indicated the possibility of digitizing the list of ingredients, with notable exceptions for allergenic substances listed in Annex III.

Customs enforcement
Presently, the EU customs' IT system operates independently of the Cosmetic Product Notification Portal (CPNP), where comprehensive product information is accessible. Compliance with the European Cosmetics Regulation necessitates product notification on CPNP before placement on the EU market. However, customs authorities face constraints in verifying product status at the border due to limited connectivity with CPNP.
Market surveillance conducted by National Competent Authorities has revealed numerous instances of non-compliance among products from non-European brands, failing to meet basic requirements outlined in the European Cosmetics Regulation. Addressing this challenge, the revised regulation will incorporate a formal linkage between CPNP and the EU customs' IT system.
This integration aims to enhance customs enforcement capabilities by providing real-time access to crucial product information stored in CPNP. By establishing this connection, customs authorities will be better equipped to identify and address non-compliant products entering the EU market, thereby bolstering consumer safety and regulatory compliance.
“Lisbonisation” of the European Cosmetics Regulation
This topic is rather technical and intends to adapt the regulation to Lisbon Treaty’s provisions. The comitology procedure currently in place and used to pass legal acts amending the annexes of the regulation will be replaced by legal instruments named “Delegated acts” and “Implementing acts”. Comitology and Delegated Acts under the Lisbon Treaty represent distinct mechanisms within the EU legislative framework, each with its own characteristics and implications.
Comitology, a set of procedures involving committees, allows EU member states to scrutinize the European Commission's adoption of non-essential changes to the regulation. This ensures member states have a say in the practical application of broad EU legislation. Historically, Comitology operated with less transparency, featuring various committee types and limited oversight. On the other hand, Delegated Acts, introduced by the Lisbon Treaty, empower the Commission to supplement or amend non-essential elements of legislative acts. This facilitates more efficient and flexible law-making on technical details. Post-Lisbon Treaty, clearer categories (delegated acts and implementing acts) were introduced, accompanied by increased parliamentary scrutiny.
As a consequence, the European Commission gains some power with Delegated Acts to fill in technical details, while having less control over implementing acts due to member state oversight. The Council and Member States retain influence over implementation through Comitology but have less control over core legislation with Delegated Acts. Finally, the European Parliament, with limited involvement in Comitology pre-Lisbon Treaty, gains greater oversight power through its ability to scrutinize Delegated Acts.
Exploring Timelines: Scenarios for the Development and Implementation of the New European Cosmetics Regulation
The timing of the release of a regulatory proposal by the European Commission holds crucial significance for the overall timeframe of the process. Gerald Renner, Technical Director of Cosmetics Europe, has outlined several scenarios:
- Early Proposal: If the regulatory proposal is issued before the European Parliament election, the current Parliament would not handle it. Instead, a Rapporteur and Lead Committee would be appointed under the new Parliament, potentially by Q4 2024. While the scope of the targeted revision would likely remain the same, new policy priorities may emerge, and significant changes could be introduced by the new Commission and Parliament. In this scenario, the legislative process would occur in 2025, with the revised regulatory framework potentially adopted by Q1 2026.
- Delayed Proposal (most likely): Alternatively, if the regulatory proposal is issued by the new Commission in Q1 2025, the targeted revision may differ from current expectations due to possibly different priorities. Subsequently, a Rapporteur and Lead Committee would be appointed in Q2 2025. Similar to the first scenario, the final regulation would heavily depend on the priorities of the new political landscape. However, the adoption of the regulation would occur later, possibly in Q4 2026.
- Ambitious Recast: A less likely scenario involves the new European Commission opting to abandon the targeted revision project in favor of a more ambitious recast. This speculative scenario could entail a complete overhaul of the European Cosmetics Regulation, introducing new principles and approaches, potentially including environmental protection measures. If confirmed, the Commission would decide on this course of action by Q1/Q2 2025. Such a profound recast would involve regulatory fitness checks, impact assessments, and public consultations, extending until end of 2027. Consequently, the Commission proposal would be issued by end of 2027, with the Ordinary Legislative Procedure continuing until end of 2028, and potential adoption of the new regulation in early 2029 at the earliest.
- Unlikely Abandonment: Renner also theorized a less probable scenario where the European Commission simply abandons the project to modernize the European Cosmetics Regulation. However, this appears almost unrealistic given the current circumstances.
These scenarios underscore the complexities and uncertainties surrounding the development and implementation of the new European Cosmetics Regulation, emphasizing the pivotal role of timing and political dynamics in shaping its trajectory.
Conclusion
In the dynamic cosmetics arena, the European Cosmetics Regulation EC N°1223/2009 has been a cornerstone of consumer protection and market integrity, emphasizing safety, transparency, and sustainability. Amid preparations for its revision, stakeholders face key decisions to enhance consumer protection and drive innovation. Changes such as updated risk assessment methods and nanomaterial definitions aim to address emerging concerns.
Voluntary digital labeling and customs enforcement enhancements highlight adaptability and efficiency in regulation, while the Lisbonisation of the regulation aims to modernize legislative processes for transparency and efficiency.
Stakeholders must stay vigilant amidst evolving regulatory timelines, understanding political dynamics. Collaboration and foresight are crucial for Europe to lead in safety, ethics, innovation, and inclusivity for consumers and society.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
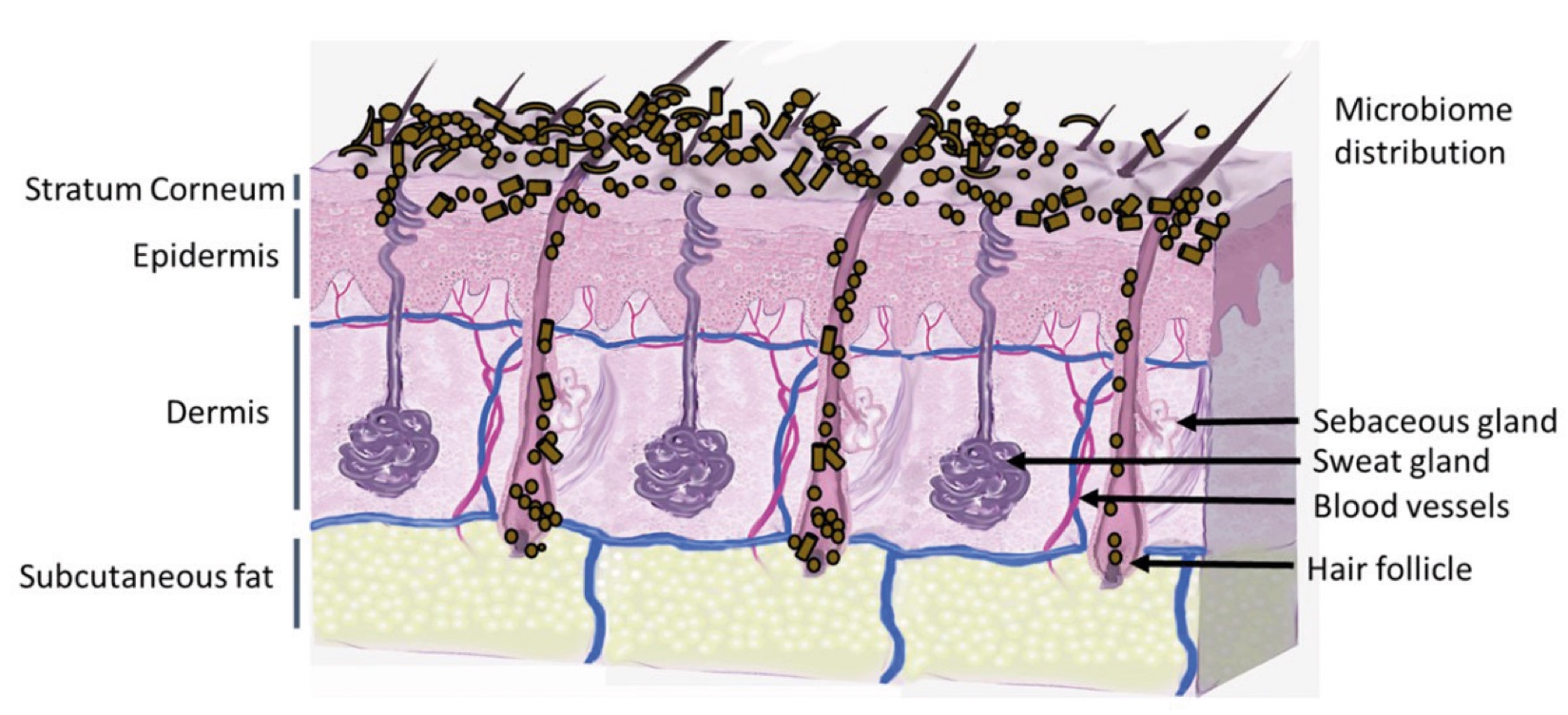
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
