
Sun care
on
Skin care
peer-reviewed
In vitro method for UVA1 (Long UVA) or Ultra-Long UVA claiming: a study based on several sunscreen products
CELINE VINCENT, SEBASTIEN MIKSA
WENEOS, Creil, France
ABSTRACT: This study highlights the critical need for effective Long UVA protection in sunscreens. Long UVA rays (340–400 nm) not only penetrate deeper than UVB but significantly contribute to premature aging, DNA damage, pigmentation issues, and an increased risk of skin cancer. Despite this, Long UVA protection is often insufficient in current formulations. Employing a robust in vitro method based on ISO 24443:2021, this research evaluates 63 sunscreens for their ability to block Long and Ultra-Long UVA rays. The findings show that only 25% of the products meet stringent criteria for Long UVA protection, pointing to an urgent need for enhanced regulatory standards and formulation improvements to better protect consumers.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
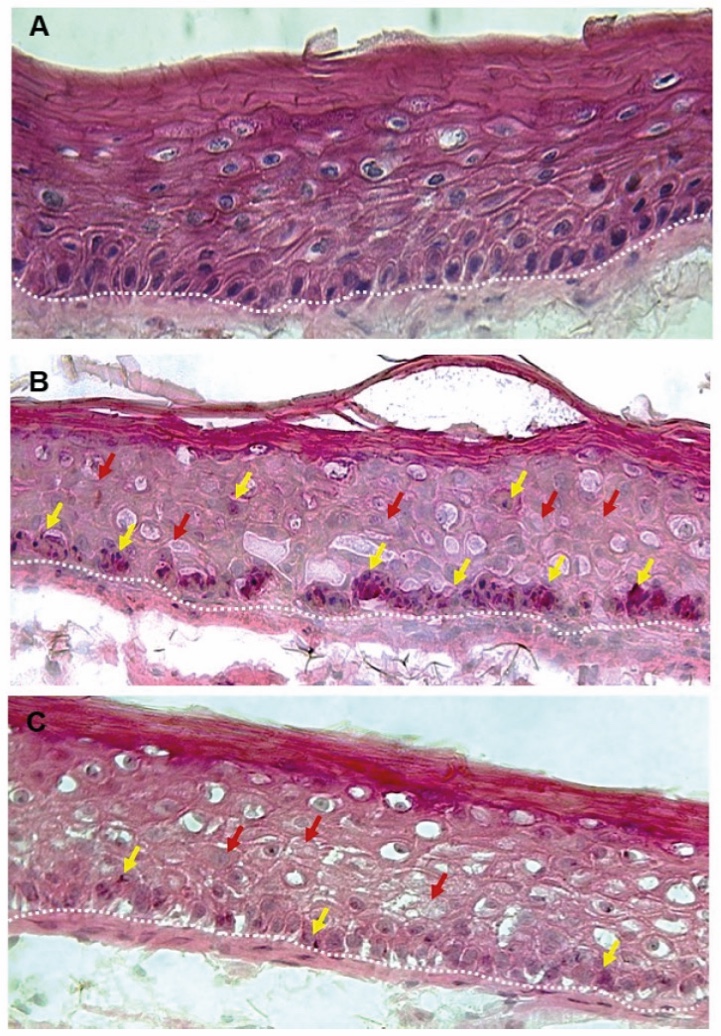
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
INTRODUCTION
In the field of sunscreen products, consumers are increasingly demanding products that not only offer superior sun protection but also additional resistances such as water resistance, friction (rub) resistance, sweat resistance, wet skin application effectiveness, and sand resistance. This indicates the growing awareness and need for comprehensive protection against the multifaceted challenges posed by sun exposure. Indeed, it is now well-known that while UVB rays are primarily responsible for sunburn and redness, UVA radiation, with its deeper penetration, is a critical factor in DNA damage, and the increased risk of skin cancer. Moreover, chronic exposure to UVA rays accelerates the aging process of the skin, characterized by wrinkles, leathery texture, and hyperpigmentation (age spots). This is due to UVA's ability to penetrate deep into the dermis, damaging collagen and other structural components of the skin. Finally, UVA radiation can cause pigmentation disorders such as melanogenesis (melanocytes produce more melanin can result in uneven pigmentation and dark spots, especially in individuals with darker skin tones), melasma (UVA exposure can worsened brown to gray-brown patches on the face) and other forms of hyperpigmentation by causing oxidative stress and inflammation in the skin.
In this way, sun protection formulations have evolved to address sensorially, transparent film and broader spectrum of ultraviolet radiation, acknowledging that both UVB (290 to 320 nm) and UVA (320 to 400 nm) rays pose significant risks to skin health. Within the specific UVA radiation, it includes UVA2 (320‒340 nm also represented by UVA-II) and UVA1 rays (340‒400 nm also represented by UVA-I) which represent for more than 80% of solar UV received on the earth. In recent years, studies have proven the harmful effects of the UVA1 in skin damages [1]-[7] while other articles highlighted the lack of sunscreen products with ultra-long UVA protection (370-400 nm) which could be solved by new UV protection including chemical or natural ingredients [8]-[11].
Nevertheless, current standardized in vitro methods [12], [13] allow the determination of the UVA Protection Factor (UVAPF), Critical Wavelength (CW) or the UVA/UVB ratio but no specific method was developed to measure in detail the Long UVA protection only. Moreover, even if regulations are available for the UVB and UVA protection for claiming purpose, the Long UVA labeling is not regulated to have a relative scale to compare products between them for consumer guidance. Beyond this point, it was scientifically difficult to express the real Long UVA protection of a sunscreen product without numbered criteria. As evidence, in vitro measurements of sunscreen strictly quantify its physical ability to block or absorb UV radiation but do not assess the biological effects of UV exposure on the skin, such as DNA damage, oxidative stress, or inflammation. Thus, while these methods indicate the sunscreen's potential physical protection, they do not capture the full spectrum of biological protection that may be incorrectly used for labelling Long UVA protection.
Therefore, the aim of the present study is the assessment and comparison of the Long UVA protection (and the Ultra-Long UVA protection) of different sunscreen products by means of a UV spectral absorbance curve from an in vitro procedure based on the referenced International Standard ISO 24443:2021. Results of this measurement procedure can be used for calculation of UVA Protection Factor I (UVA-PF-I), Critical Wavelength (CW) and UVA-I/UV ratio. Finally, a pass/fail criterion is proposed from the global comparison of sunscreen products to effectively claim a Long UVA protection or Ultra-Long UVA protection.
MATERIALS AND METHODS
- Sunscreen product: Sixty-three products with different properties and cosmetic presentations were selected (Table 1). These are different forms including mainly emulsion (tinted or not) but also stick, biphasic and alcoholic spray, with various levels of protection ranging from SPF 15 to 50+. Furthermore, the selected products include reference standards (S2, P2, P5 and P8), classical sunscreen products without the Ultra-Long UVA label on the packaging and sunscreen products from the market with the Ultra-Long UVA label on the packaging (a total of 6 products).
- Substrate: The importance of substrate roughness on the reproducibility of in vitro SPF tests has already been demonstrated (intensity and shape of the curve). For the described tests, substrates used were molded polymethylmethacrylate (PMMA) plates (Helioplate HD6, WENEOS ex HelioScreen) and sandblasted plates (Helioplate SB6, WENEOS ex HelioScreen), for spectrophotometric measurements from 290 nm to 400 nm. These plates are in total compliance with the specifications described in the ISO 24443:2021 for in vitro UVA-PF determination for transmittance ([290 nm: >60 %T]; [300 nm: >69 %T]; [320 nm: >81 %T]) and roughness (several topographic parameters).
- Application: Before testing, products were stored at a controlled temperature for 24 hours were shaken just before their application to ensure a good homogenization. To obtain the correct application rate of 1.3 mg/cm² (HD6) or 1.2 mg/cm² (SB6), at least nine drops of the tested sunscreen product were applied by an automatic pipette (Multipette E3, EPPENDORF) with Combitips Advanced 100 µl across the entire PMMA plate.
- Automated spreading: Immediately after weighing, the sunscreen was spread using an automated device (HD-SPREADMASTER, WENEOS ex HelioScreen) which is a robotic arm performing precise and repeatable circular and linear strokes with a control of the pressure (compliant with the ISO 24443:2021). First, the product is spread on the whole area of the plate, using circular movements with a minimum of four passages from the top to the bottom of the plate. At the end of the first passage, a turn of the plate has to be done (¼ turn) to alternate passages and repeat this movement three times at least (about 30 s). Then the sample is rubbed on the plate surface using alternating horizontal and vertical strokes repeated at least three times alternate passages (about 30 s).
- Drying time: After automated spreading, the sunscreen was allowed to dry and settle at 27°C ± 2°C for at least 30 minutes (i.e. in compliance with the ISO 24443:2021 indicating a drying for 30 min to 60 min in the dark between 27°C ± 2°C and 32°C ± 2°C). This step is necessary to consider the relaxation of tested products. During this drying process, the temperature was maintained and controlled by means of a device (HD-THERMASTER, WENEOS ex HelioScreen).
- Transmittance measurement: Once the drying step was completed, it is now necessary to measure the UV transmittance spectrum through the thin irregular layer of sunscreen product spread on the substrate. The concept of transmittance corresponds to the quantity of UV transmitted through the sunscreen product evaluated for a single wavelength. This notion is linked to the absorbance which corresponds to the quantity of UV absorbed by the product.
The evaluation of sunscreen product’s absorbance was performed using a spectrophotometer from 290 to 400 nm (UV-2000S, Labsphere Inc.). Before taking measurements, the transmittance analyzer was validated and controlled according to the ISO 24443:2021 method including the linearity/additivity by calibrated reference standard PMMA calibration plates (Helioplate HD0, WENEOS ex HelioScreen) and Holmium Oxide filter for wavelength accuracy. Moreover, a PMMA plate covered with a film of white petrolatum (15 mg applied) was used to obtain blank transmittance.
Four plate replicates were used for each tested product, and nine UV transmission spectra were recorded for each plate at the different application locations (i.e. a total of 27 UV spectra). These steps were repeated before and after irradiation.
- Adjustment of UV spectrum: Following the ISO 24443:2021 principle, the first adjustment consists in fitting the in vitro SPF calculated from the measured UV spectrum (Equation 1)with the in vivo SPF value. For this purpose, the initial absorbance curve was multiplied by a correction factor C until the in vitro calculated SPF is equal to the measured in vivo SPF according to an iterative calculation process (Equation 2). The choice between the molded PMMA plates HD6 and the sandblasted PMMA plates SB6 was determined by the C factor that was closer to 1.
Equation 1:
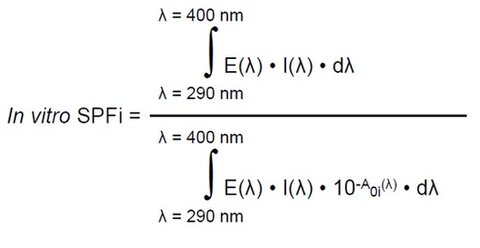
E(λ) = Erythema action spectrum (CIE-1987).
I(λ) = Spectral irradiance received from the UV source (UV-SSR for SPF testing).
A0i(λ) = Mean monochromatic absorbance of the test product layer before UV exposure.
dλ = Wavelength step (1 nm).
Equation 2:
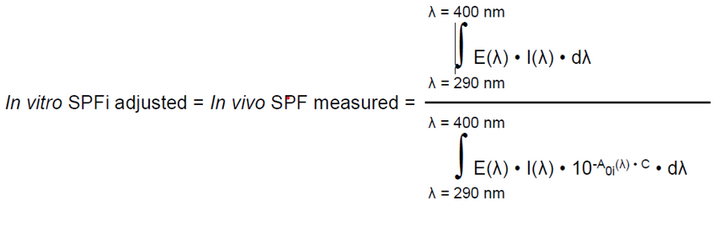
where:
E(λ), I(λ), A0i(λ) and dλ previously determined in Equation 1.
C = Coefficient of adjustment.
- UV exposure: To consider a potential photodegradation of tested products, it is necessary to perform an irradiation step. For this purpose, a solar simulator compliant with ISO 24443 specification (Model LS1000-4S-009, Solar Light Company, Inc. or Suntest CPS, AMETEK+) was used to expose samples. Moreover, prior to each UV exposure, the intensity of the UV source used for exposure was checked using either a spectroradiometer or a calibrated radiometer with sensitivity in the UVA.
The in vitro UVAPF0i is firstly calculated (Equation 3) and then used for the pre-irradiation dose D required for the UV exposure (Equation 4).
Equation 3:
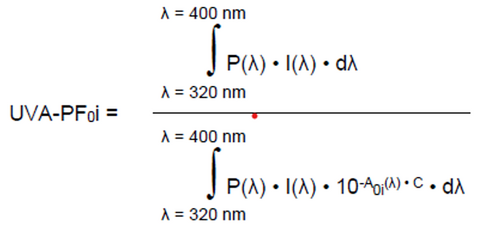
where:
P(λ) = PPD action spectrum.
I(λ) = Spectral irradiance received from the UVA source (UVA 320-400nm for PPD testing)
A0i(λ), dλ and C previously determined in Equation 1 and 2.
The pre-irradiation dose D required for UV exposure has been calculated with the (Equation 4) and should be limited at a maximum of 36 J/cm².
Equation 4:

- Calculation of the Long UVA factors: Based on the adjusted absorbance spectrum, several factors are calculated to represent the potential of Long UVA protection.
a. In vitro UVAPF-I and UVAPF-I/Labelled SPF ratio: The final UVAPF-Ii is calculated according to Equation 5 including the UV absorbance value after UV exposure (Ai(λ)) for the same plate and the UVAPF-I is equal to the arithmetic mean of all valid individual UVAPF-Ii values obtained from all tests performed and expressed as a whole number. Contrary to the initial wavelength range of 320 to 400 nm, it was decided to limit the range to 340 to 400 nm to specifically consider the long UVA rays only, thereby avoiding the over-consideration of short UVA protection in the UVA-PF value.
Equation 5:
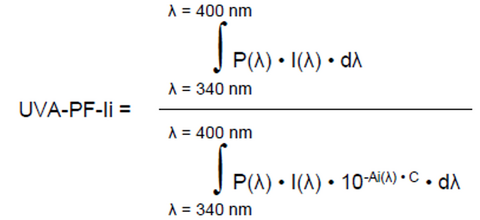
where:
P(λ), I(λ) are defined in Equation 3 but limited from 340 to 400 nm.
Ai(λ) = Mean monochromatic absorbance of the test product layer after UV exposure.
dλ and C previously determined in Equation 1 and 2.
The UVAPF-I/Labelled SPF ratio is an indicator of UVAPF-I properties of a sunscreen product, compared to SPF properties according to Equation 6. To have a good UVA absorption, the UVAPF-I has to be at least a third (1/3) of the labelled SPF. The final UVAPF-I/Labelled SPF ratio is the in vitro UVAPF-I obtained by this method on the labelled SPF.
Equation 6:

where:
UVAPF-I has been obtained by the present in vitro method.
Labelled SPF has been determined following local regulation.
b. Critical Wavelength (CW): The individual CWi (λci) is defined as the wavelength at which the area under the absorbance curve (Ai(λ)) of the product from 290 nm is equal to 90% of the integral of the absorbance curve from 290 nm to 400 nm of the same plate after the UV exposure at the D dose according to Equation 7. The final CW (λc) value is the arithmetic mean of all valid individual CWi (λci) values obtained from all tests performed.
Equation 7:
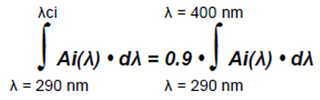
where:
Ai(λ) = Mean monochromatic absorbance of the test product layer after UV exposure.
λci = Wavelength at which the area under the absorbance curve (A(λ)) of the product from 290 nm is equal to 90% of the integral of the absorbance curve from 290 nm to 400 nm.
c. UVA-I/UV ratio value : The UVA-I/UVi ratio (individual UVA-I/UV ratio) is an indicator of Long UVA absorption properties (area per wavelength unit in the UVA-I portion of the absorbance curve) of a sunscreen product, compared to UV absorption properties (area per wavelength unit in the UV portion of the absorbance curve) of the one and same plate after the UV exposure at the D dose, rather than a ratio of transmission or protection factors according to Equation 8. The final UVA-I/UV ratio is the arithmetic mean of all valid individual UVA-I/UVi ratio values obtained from all tests performed.
Equation 8:
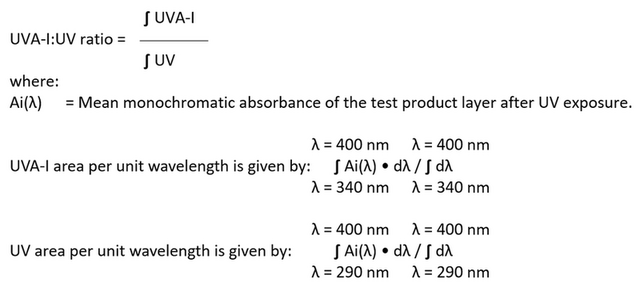
RESULTS AND DISCUSSION
The aim of the present study is the analysis of the Long UVA (Ultraviolet-A) rays protection of sixty-three sunscreen products by means of a UV spectral absorbance curve from an in vitro procedure based on the referenced International Standard ISO 24443:2021 principle. To reach this goal, it was necessary to define a pass/fail criterion to highlight sunscreen products with a significant Long UVA protection based on all results of UVA Protection Factor I (UVAPF-I), Critical Wavelength (CW) and UVA-I/UV ratio.
For the first criterion, based on the COMMISSION RECOMMENDATION of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto [14] from European Union, we decided to use the same minimum UVAPF/Label SPF ratio of 1/3 but extended to the long UVA (expressed by the UVAPF-I/Label SPF ratio).
For the second criterion, we decided to represent the UVAPF-I/Label SPF versus the Critical Wavelength (Figure 1) to detect the minimum Critical Wavelength necessary to respect the 1/3 criteria (UVAPF/Label SPF ratio) as requested above. Based on the Figure 1 and by using the linear trend line, it is possible to observe that a Critical Wavelength of at least 375 nm (rounding up value) is requested to respect a UVAPF-I/Label SPF criterion of 1/3.
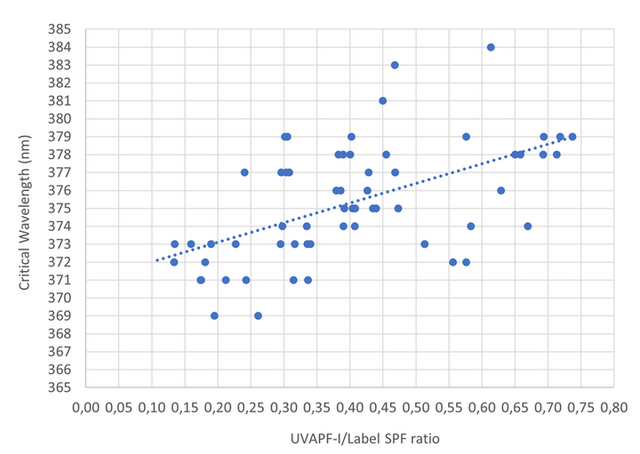
Figure 1. UVAPF-I/Label SPF ratio versus Critical Wavelength.
For the third criterion, we decided to represent all UVA-I/UV ratio values in a box plot (Figure 2) to determine a minimum protection to be afforded by a sunscreen product to claim the “Long UVA” protection. Based on the Figure 2, it is possible to observe that a UVA-I/UV ratio of at least 0.85 represents the median of all products evaluated and this criterion was, therefore, retained to only select sunscreen products with the highest UVA-I protection.
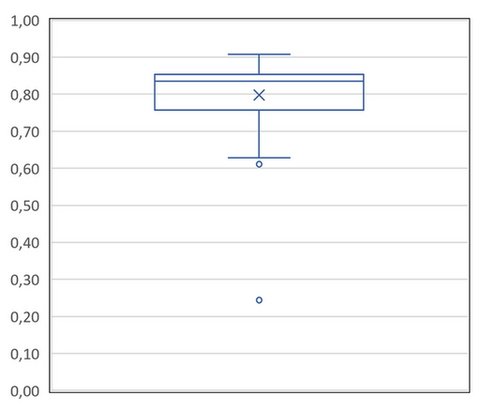
Figure 2. UVA-I/UV ratio box plot.
Therefore, based on these different above steps, it is noted that a product can claim “Long UVA” protection if all these following criteria are passed:
- UVAPF-I/Labelled SPF ratio ≥ 1/3
- CW ≥ 375 nm
- UVA-I/UV ratio ≥ 0.85
Finally, by using these three criteria simultaneously with all tested sunscreen products in the present study, it is possible to observe that the selected selectivity is about 75% (15 products pass the criteria and forty-eight products fail the criteria), i.e., 1 product in 4 can claim the Long UVA protection. Moreover, it is interesting to observe that only about half of the sunscreen products from the market with the Ultra-Long UVA label on the packaging pass all the three criteria.
Furthermore, we observed that a specific brand, a specific galenic (between emulsion, biphasic, stick, etc.), the labelled SPF level, and a specificity (tinted or not) are not the critical factor influencing the pass/fail criteria (with a percentage about 25% between cases).
The illustrations of the requested absorbance curves necessary to have a Pass conclusion or a Fail conclusion are represented in the Figure 3. From these curves, it is possible to observe that beyond the UVA-II impact (short UVA protection), the Pass conclusion is possible by the integration of the UVA-I protection but also with the consideration of the ultra-long UVA protection (370 - 400 nm).
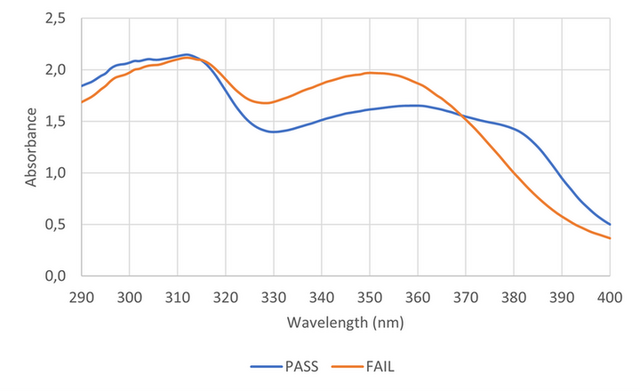
Figure 3. Absorbance curves for sunscreen products with a PASS/FAIL Long UVA protection.
CONCLUSION
Currently, the harmful effects of an overexposure to the sun are worldwide recognized and are focused on the UVB and UVA damages. As the solar spectrum is continuous electromagnetic waves, previous studies expressed the need for a protection beyond the UV rays with a Full Spectrum protection and which can be divided in different ranges such as the Blue Light, Visible Light, and Infrared protection. In addition, recent studies helped to understand the need to extend the protection in the UV range and more especially in the Long UVA and Ultra-Long UVA spectrum.
Based on the comparison of 63 sunscreen products, this study presents the development and the results of a new in vitro method with specific criteria to assess the Long UVA protection or Ultra-Long UVA protection provided by sun care products. Indeed, according to different results, the three criteria proposed to highlight products with a significant highest Long UVA protection were (i) UVAPF-I/Labelled SPF ratio ≥ 1/3, (ii) CW ≥ 375 nm and (iii) UVA-I/UV ratio ≥ 0.85.
To conclude, from these criteria, it was interesting to observe that one product in four can be positively evaluated to the UVA long protection (also called Ultra-Long UVA protection) and could be claimed accordingly.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
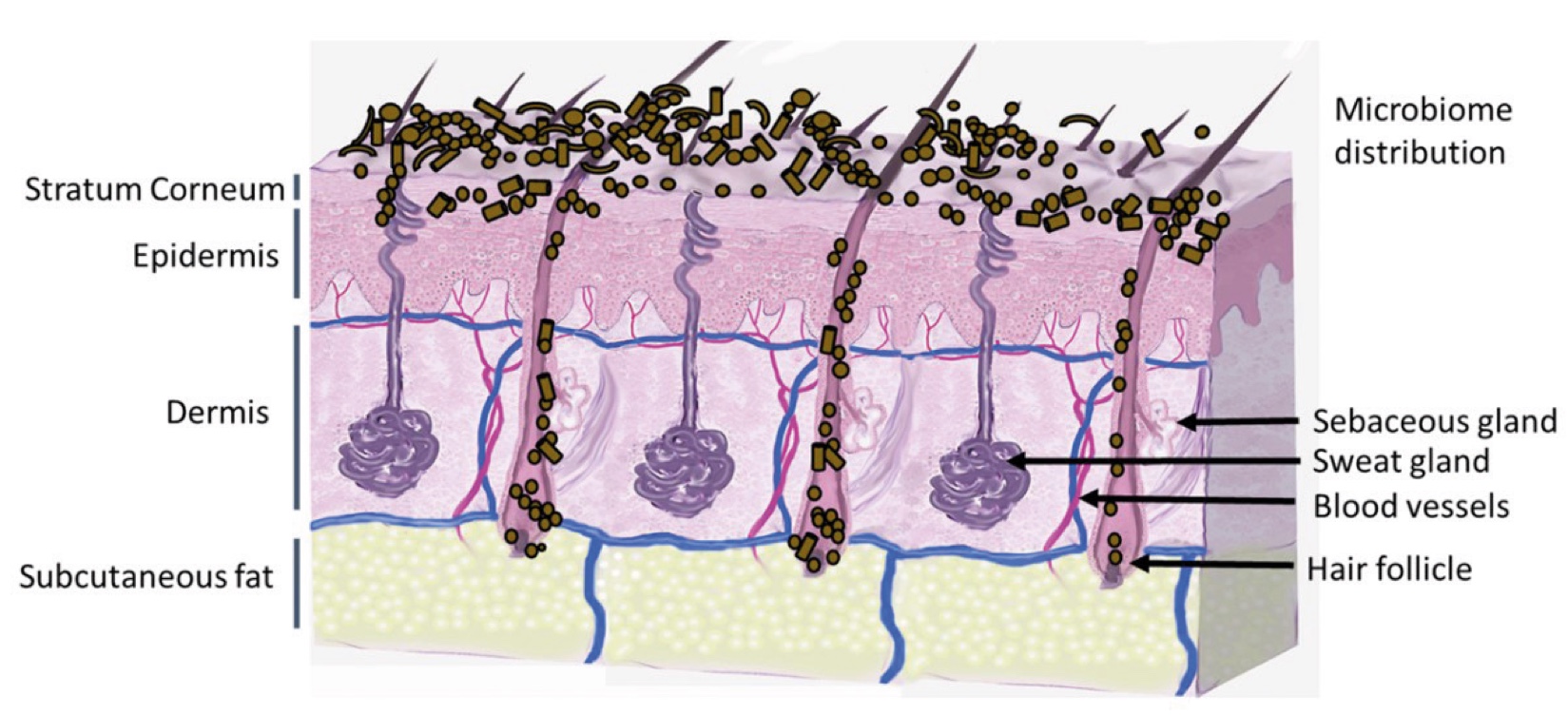
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Tewari A, Grage MM, Harrison GI, Sarkany R, Young AR. UVA1 is skin deep: molecular and clinical implications. Photochem Photobiol Sci. 2013 Jan;12(1):95-103. doi: 10.1039/c2pp25323b. PMID: 23192740.
- Wang F, Smith NR, Tran BA, Kang S, Voorhees JJ, Fisher GJ. Dermal damage promoted by repeated low-level UV-A1 exposure despite tanning response in human skin. JAMA Dermatol. 2014 Apr;150(4):401-6. doi: 10.1001/jamadermatol.2013.8417. PMID: 24305962; PMCID: PMC4167395.
- Marionnet C, Pierrard C, Golebiewski C, Bernerd F. Diversity of biological effects induced by longwave UVA rays (UVA1) in reconstructed skin. PLoS One. 2014 Aug 20;9(8):e105263. doi: 10.1371/journal.pone.0105263. PMID: 25140898; PMCID: PMC4139344.
- Marionnet C, Nouveau S, Hourblin V, Pillai K, Manco M, Bastien P, Tran C, Tricaud C, de Lacharrière O, Bernerd F. UVA1-Induced Skin Darkening Is Associated with Molecular Changes Even in Highly Pigmented Skin Individuals. J Invest Dermatol. 2017 May;137(5):1184-1187. doi: 10.1016/j.jid.2016.12.016. Epub 2017 Jan 10. PMID: 28082185.
- Marionnet C., Tran C., Bastien P, Bielicki A., Golebiewski C., Vieu D. Suida-Batista A., Candau D., Bernerd F., A broader filtration of UVA1 wavelengths improves skin photoprotection, Journal of Dermatological Science, VOLUME 91, ISSUE 3, P337-340, SEPTEMBER 2018. doi: 10.1016/j.jdermsci.2018.06.008.
- Kohli, I., Zubair, R., Lyons, A.B., Nahhas, A.F., Braunberger, T.L., Mokhtari, M., Ruvolo, E., Lim, H.W. and Hamzavi, I.H. (2019), Impact of Long-Wavelength Ultraviolet A1 and Visible Light on Light-Skinned Individuals. Photochem Photobiol, 95: 1285-1287. doi: 10.1111/php.13143.
- Aguilera J, Gracia-Cazaña T, Gilaberte Y. New developments in sunscreens. Photochem Photobiol Sci. 2023 Oct;22(10):2473-2482. doi: 10.1007/s43630-023-00453-x. Epub 2023 Aug 5. PMID: 37543534.
- Marionnet Cde Dormael RMarat X et al. Sunscreens with the New MCE Filter Cover the Whole UV Spectrum: Improved UVA1 Photoprotection In Vitro and in a Randomized Controlled Trial, JID Innovations, ISSN: 2667-0267, Vol: 2, Issue: 1, Page: 100070, DOI: 10.1016/j.xjidi.2021.100070.
- Zhang X, Tao H, Zhang Z, Wang W, Steel A, Fang X, He X. Evaluation of the efficacy of a sunscreen containing ultra-long UVA1 and other UVR broad-spectrum filters on skin barrier protection and melanin content reduction in Chinese adults: A single-center study. Health Sci Rep. 2024 Feb 22;7(2):e1923. doi: 10.1002/hsr2.1923. PMID: 38405170; PMCID: PMC10884559.
- Flament F, Mercurio DG, Muller B, Li J, Tricaud C, Bernerd F, Roudot A, Candau D, Passeron T. The impact of methoxypropylamino cyclohexenylidene ethoxyethylcyanoacetate (MCE) UVA1 filter on pigmentary and ageing signs: An outdoor prospective 8-week randomized, intra-individual comparative study in two populations of different genetic background. J Eur Acad Dermatol Venereol. 2024 Jan;38(1):214-222. doi: 10.1111/jdv.19486. Epub 2023 Sep 12. PMID: 37655436.
- Ezekwe N, Pourang A, Lyons AB, Narla S, Atyam A, Zia S, Friedman BJ, Hamzavi IH, Lim HW, Kohli I. Evaluation of the protection of sunscreen products against long wavelength ultraviolet A1 and visible light-induced biological effects. Photodermatol Photoimmunol Photomed. 2024 Jan;40(1):e12937. doi: 10.1111/phpp.12937. Epub 2023 Dec 8. PMID: 38069506.
- ISO 24443:2021 Cosmetics-sun protection test method - Determination of the UVA Photoprotection in vitro
- US FDA, Department of Health and Human Services. Sunscreen drug products for over-the-counter human use, Final Rule 21 CFR parts 201 and 310, Federal Register 76(117) (2011).
- European C4ommission Recommendation 2006/647/EC.

