
Regulation
on
Skin care
peer-reviewed
Substances on the spotlight
SYBILLE MILLET
Regulatory affairs manager, COSMED, France
Article received on March 8, 2024
ABSTRACT: Regulations are changing fast. Every year, new substances are restricted or banned. Cosmed takes a look at the many initiatives underway that are having an impact on cosmetics.
??????????????????
“
“A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans”
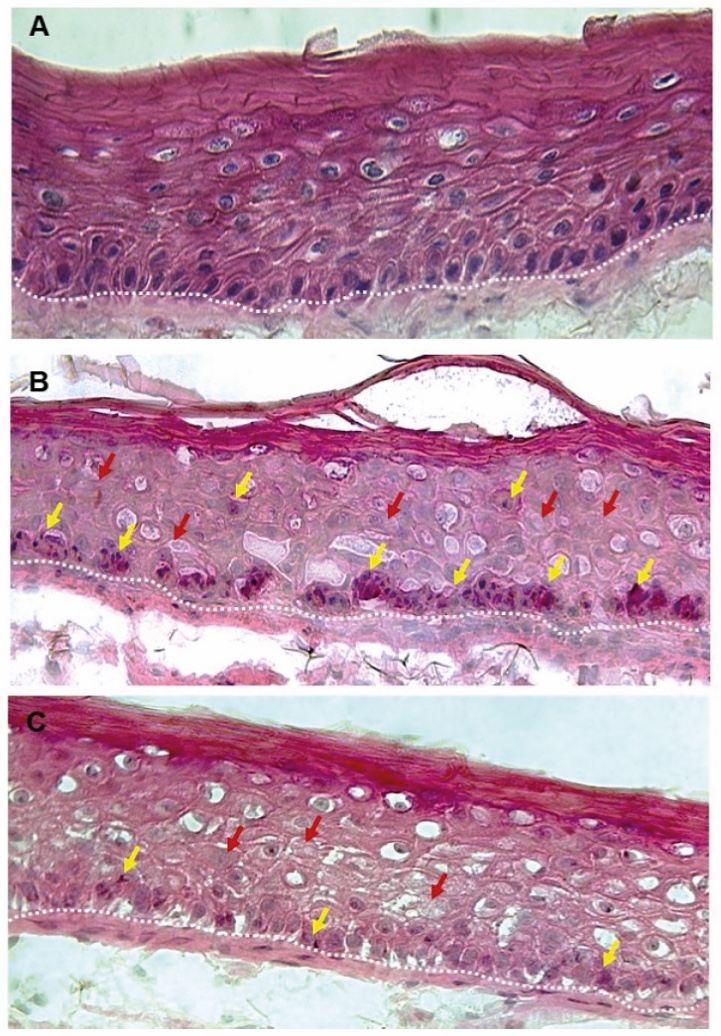
Figure 1. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
Materials and methods
Studies of major depressive disorder have been correlated with reduced Lactobacillus and Bifidobacteria and symptom severity has been correlated to changes in Firmicutes, Actinobacteria, and Bacteriodes. Gut microbiota that contain more butyrate producers have been correlated with improved quality of life (1).
A study in healthy women providing probiotic yogurt for four weeks showed an improvement in emotional responses as measured by brain scans (2). A subsequent study by Mohammadi et al. (3) investigated the impacts of probiotic yogurt and probiotic capsules over 6 weeks and found a significant improvement in depression-anxiety-stress scores in subjects taking the specific strains of probiotics contained in the yogurt or capsules. Other studies with probiotics have indicated improvements in depression scores, anxiety, postpartum depression and mood rating in an elderly population (4-7).
Other studies have indicated a benefit of probiotic supplementation in alleviating symptoms of stress. In particular, researchers have looked at stress in students as they prepared for exams, while also evaluating other health indicators such as flu and cold symptoms (1). In healthy people, there is an indication that probiotic supplementation may help to maintain memory function under conditions of acute stress.
Introduction
In the context of the green deal and its chemical strategy, the heart of the chemical regulations is being revised. In parallel of these large-scale projects, regulations on substances continue to be introduced. 2023 has been busy, 2024 will remain intense. This paper is presenting some substances in scope of intentions or new regulations.
CMR: clear process with variable impacts
CMR substances (carcinogenic, mutagenic and reprotoxic) are subject to a process of prohibition and authorisation under relatively clear exemption conditions, governed by Article 15 of the Cosmetics Product Regulation and implemented through the publication of the OMNIBUS regulation, at an annual frequency, since 2019.
Omnibus VII is being prepared to include substances classified as CMR under ATP 21 of the CLP (Classification, Labelling and Packaging). This Omnibus is considered to have low impact, with the ban of substances in Annex II with a limited use and not defended (DIMETHYLTOLYLAMINE and TRIMETHYLBENZOYL DIPHENYLPHOSPHINE OXIDE). However, other classifications currently under development are expected to have a much greater impact. In particular, draft ATP 22 includes 3 substances used in cosmetics and defended by industry:
- hexyl salicylate, with the intention to classify it as CMR, toxic for reproduction category 2. This substance is used in fragrances and is already subject to a favourable preliminary opinion.
- silver, with the intention to classify it as CMR, toxic for reproduction category 2. Micron-sized particulates silver are the subject of a defence dossier.
- o-phenylphenol, which is intended to be classified as a category 2 carcinogen (CMR). This is a preservative listed in Annex V of the regulation it will also be defended.
These classifications should come into force at the end of 2025/beginning of 2026.
Natural substances are not spared from CMR classification. For example, cannabidiol (CBD), tea tree oil and p-cymene, a component of many essential oils, are also subject to classification.
- CBD has been the subject of an intention to classify as a reprotoxic substance published by the ECHA on 6 June 2023. This dossier, submitted by France, should be open for comment in 2024. In the absence of data submitted by the industry demonstrating its safe use, CBD could be banned in cosmetics.
- Tea tree oil is currently being classified as CMR, toxic for reproduction category 1B, with the intention of including it in ATP 23 of the CLP regulation. This classification as CMR category 1B makes its defence much more difficult. COSMED has set up a task force to defend the safe use of this substance in cosmetics. Depending on the outcome of the discussions, the fate of this substance (authorised or banned) could be sealed around July 2026.
- p-Cymene is in scope of an intention to classify it as CMR 1B. It is a component of a very large number of essential oils used in cosmetics. The classification of p-cymene as CMR 1B will have an impact on the use of essential oils containing it. Discussions are planned at EU cosmetics WG level to consider the case of essential oils and the implementation of a rational approach to study cases of constituents classified as CMR 1B.
Nanomaterials: work in progress before the major one
The EU Commission has introduced the Nano Omnibus regulations to transcribe SCCS opinions into the annexes of the regulation and to ban substances for which the opinion has been inconclusive. The first Nano Omnibus was published on 15 March 2024 and is adding 5 entries to the annex of prohibited substances in the cosmetics regulation:
- Styrene/acrylate copolymer [nano] and Sodium Styrene/Acrylate copolymer [nano].
- Copper [nano] and Colloidal copper [nano]
- Colloidal silver [nano]
- Gold (nano) / Colloidal Gold (nano) / - Gold Thioethylamino Hyaluronic Acid (nano) / Acetyl
- heptapeptide-9 Colloidal gold (nano)
- Platinum (nano) and Colloidal Platinum (nano) / - Acetyl tetrapeptide-17 Colloidal Platinum (nano).
In addition to these bans, Hydroxyapatite[nano] is added to annex III of restricted substances following the favourable opinion of SCCS (1)
Another work is expected following the publication of the European Commission's recommendation no. 2022/C229/01 on the new definition of "nanomaterial" in June 2022. The cosmetics regulation will be aligned with this definition. However, we will have to wait for the revision of the cosmetics regulation before this becomes official. This new definition will have an impact because the loss of the intentionality criterion means that many substances currently excluded from the cosmetic definition will have to be considered as nano. This will have a particular impact on many natural colourants.
UV filters under pressure
Although it is essential to maintain a wide palette of UV filters to guarantee the effectiveness of sun protection products, they are currently subject to unprecedent regulatory pressure. While Annex VI of the cosmetics regulation includes 31 INCI names (in nano or non-nano form), in practice the number of filters used is much smaller.
Firstly, from a regulatory point of view, several UV filters are currently being assessed.
The list of 28 substances identified in 2019 as priorities for assessment because of concerns about their potential endocrine effects included 7 UV filters.
- Safe use has been confirmed by the SCCS at a given concentration for Benzophenone-3 and Octocrylene (2) (3).
- Reformulations are underway following the restriction on the use of Homosalate (4), authorised only in facial products, at a reduced concentration with application dates in 2025.
- 4-MBC, which had already been ruled out by the industry, will be banned by a regulation to be published in 2024, with a deadline of 2025.
- Benzophenone-4 is currently being assessed, with a positive preliminary SCCS opinion.
- Ethylhexyl methoxycinnamate is subject to a mandate adopted on 28 February 2024, with a 9-month deadline for its assessment by the SCCS.
- Benzophenone-5 will also be subject to a mandate from the SCCS.
In addition, there is currently a call for data on salicylate esters. This will potentially impact homosalate and ethylhexyl salicylate. While it is still too early to draw conclusions of this call for data, it remains to be followed closely.
More recently, France intends to restrict the use of Octocrylene in cosmetic products because of its potential effects on the environment. In this context, Cosmed took part in the call for data, stressing the importance of considering the global regulatory context and the need to maintain a choice of UV filters to formulate suncare with pleasant sensorially that encourage proper application key to protect the skin against the damaging effects of sun. In addition, environmental risk assessment methods need to be refined and adapted to the types of products concerned before final conclusions can be drawn.
Development of environmental restrictions
Environmental restrictions are tremendously increasing with the objective to better protect the environment. Some regulations have been published or are in the process of being developed under the REACH regulation that affect cosmetics.
Firstly, Regulation (EU) 2023/2055 on microplastics, published on 26 September 2023, adds a new entry to Annex XVII of the REACH regulation. It aims to phase out synthetic polymers that meet the definition of microplastics and are intentionally added to all consumer products, including cosmetics.
ECHA has defined microplastics as synthetic microparticles in solid form composed of polymers less than 5 mm in size, insoluble, containing carbon atoms, non-biodegradable or that are not the result of a polymerisation process that has taken place in nature. This regulation applies in a phase manner according to product categories: four years for rinsed products, six years for non-rinsed products, and 12 years for make-up. While exemption criteria exist, this regulation introduces reporting by 2027 and labelling requirements within 2 years. The aim of this regulation is to control the use of microplastics throughout the supply chain, up to the point of use by the consumer, in order to avoid releases into the environment. The definition given in the regulation does not allow to establish a list of microplastics, the approach to identify them should consist in looking at the characteristics of each microplastic and their use. The raw materials portfolio and labelling requirements are currently being assessed by the different stakeholders.
Another restriction concerns 3 silicones with the Regulation (EU) 2024/1328 published on 16 May 2024. Cyclotetrasiloxane (also known as D4) is already banned in cosmetics, but the impact is greater for cyclopentasiloxane (also known as D5) and cyclohexasiloxane (also known as D6). This restriction l limits them to 0.1%:
- in non-rinsed products for D5 and D6 from 6 June 2027,
- in rinsed products for D6 from 6 June 2026 (D5 is already restricted in rinsed products).
Another intention to restrict PFAS is in the pipeline. This acronym stands for per- and polyfluoroalkylated substances, which are used for a wide range of purposes: as emulsifiers, antistatic agents, stabilisers, surfactants, film-forming agents and viscosity enhancers. The European Commission's Cosing database lists no fewer than 170 of these substances in cosmetics. These substances are controversial because of their harmful impact on the environment and their potential impact on health. The European Chemicals Strategy for Sustainability aims to eliminate them.
The PFHxA subgroup (undecafluorohexanoic acid) has already been subject to a draft regulation due to be adopted in 2024. It will not have a direct impact on the cosmetics sector, but manufacturers must remain vigilant, as PFHxA may be present as an impurity and/or as a product of the degradation of intentionally added PFAS. Another restriction is currently being defined for PFAS, supported by 5 Member States (Germany, Denmark, Netherlands, Norway, Sweden). Due to the ubiquitous use of PFAS, the first consultation launched in September 2023 on ECHA’s website received a record number of contributions. This proposal for a restriction (5) on the manufacture, placing on the market and use of PFASs remains valid and is currently being reworked. Cosmetics will be one of the priority sectors. To be continued...
SCCS opinion: a way to anticipate future restrictions
It remains key to follow the SCCS evaluations in order to anticipate future insertions, modifications or withdrawals into the annexes of the cosmetic regulation. The regulation (EU) n°2024/996 was published on 4 April 2024 to introduce new provisions on vitamin A, arbutin, genistein, deidzein, BHT, triclosan and triclocarban in line with the opinions of the SCCS plus a ban for the 4-MBT. Then, the final opinion on aluminium (submission IV) will re-launch the debate in the European Commission's working group.

Conclusion
The regulatory environment continues to evolve at a rapid pace with multiple parallel regulations moving forward under the ultimate goal from the green deal to ensure the safety of consumers and the environment. The need for a 360° view remains a challenge for regulatory departments to understand the coming changes, assess their portfolio and activate relevant changes to anticipate the new requirements. Cosmed undertakes an active regulatory watch to support and guide its members and maintains a continuous dialogue with the French and European authorities to advocate for relevant applicable restrictions with realistic timetables.
Surfactant Applications

The application area lends itself particularly well to the use of AI. Active today in this area is the US company Potion AI (6). The company provides AI-powered formulation tools for beauty and personal care R&D. Their offerings include Potion GPT, next generation ingredient and formula databases and AI document processing. Potion’s work could have a significant impact on the entire surfactant value chain, from raw material suppliers to end consumers. By using their GPT technology, they can help target work toward novel surfactant molecules that have optimal properties for specific applications. By using their ingredient and formula databases, they can access and analyze a vast amount of data on surfactant performance, safety, and sustainability. By using their AI document processing, they can extract and organize relevant information from patents, scientific papers, and regulatory documents. These capabilities could enable Potion AI's customers to design and optimize surfactant formulations that are more effective, eco-friendly, and cost-efficient. A particularly interesting application for this type of capability is deformulation.
Deformulation is the process of reverse engineering a product's formulation by identifying and quantifying its ingredients. Deformulation can be used for various purposes, such as quality control, competitive analysis, patent infringement, or product improvement. However, deformulation can be challenging, time-consuming, and costly, as it requires sophisticated analytical techniques, expert knowledge, and access to large databases of ingredients and formulas.
AI can potentially enhance and simplify the deformulation process by using data-driven methods to infer the composition and structure of a product from its properties and performance. For example, AI can use machine learning to learn the relationships between ingredients and their effects on the product's characteristics, such as color, texture, fragrance, stability, or efficacy. AI can also use natural language processing to extract and analyze information from various sources, such as labels, patents, literature, or online reviews, to identify the possible ingredients and their concentrations in a product.
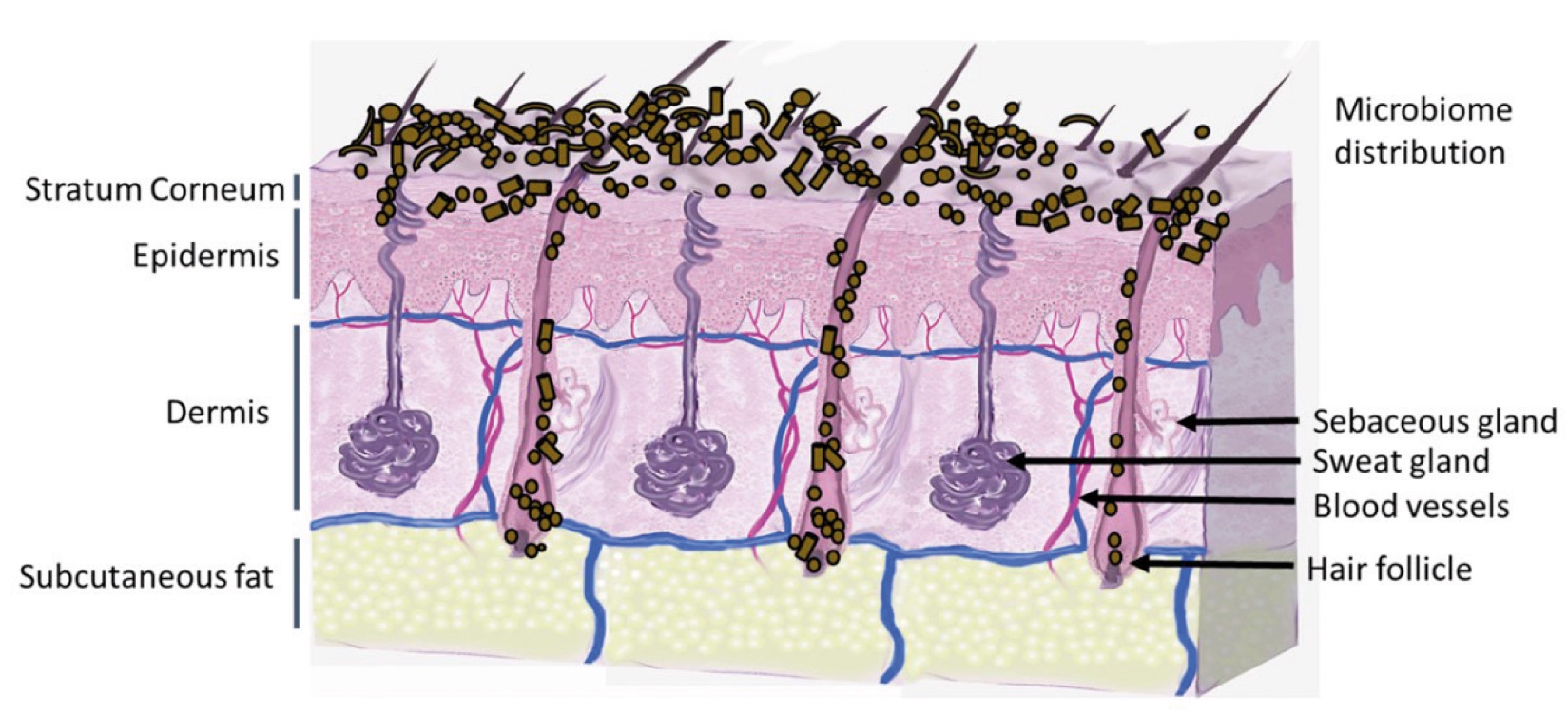
Figure 2. Skin Section with Microbiome. Most microorganisms live in the superficial layers of the stratum corneum and in the upper parts of the hair follicles. Some reside in the deeper areas of the hair follicles and are beyond the reach of ordinary disinfection procedures. There bacteria are a reservoir for recolonization after the surface bacteria are removed.
References and notes
- Opinion SCCS/1648/22 on hydroxyapatite [nano] adopted on 21/22 March 2023
- Opinion SCCS/1625/20 on Benzophenone-3 adopted on 30/31 March 2021
- Opinion SCCS/1627/21 on Octocrylene adopted on March 2021
- Regulation (UE) n°2022/2195 published on 11 November 2022
- Annex XV Restriction Report on Per- and polyfluoroalkyl substances (PFAS)
